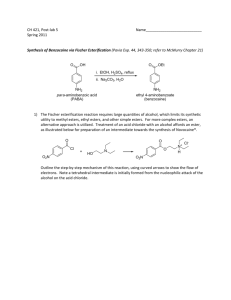
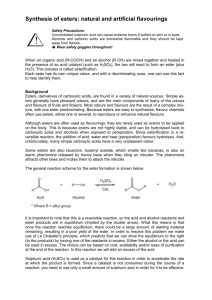
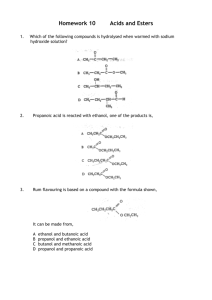
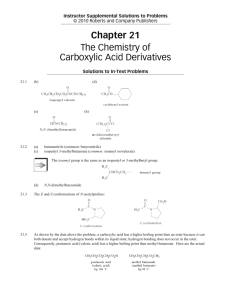
Reactions of esters:
advertisement

Organic chemistry and Biological chemistry for Health Sciences 59-191 Lecture 12 Reactions of esters: In the presence of acid catalyst, esters react with water to give alcohol and carboxylic acids. In vivo, enzymes perform the same reaction. This reaction is called hydrolysis. Ester bond is broken in hydrolysis reaction. Dietary fats and oils are digested through this hydrolysis reaction. The molecules of fats and oils-triglycerol have three ester groups per molecule. A different enzyme cleaves each ester bond. If an aqueous base instead of a strong acid is used to promote the breakup of the ester, the products are the salts of the parent acid and the parent alcohol. The reaction is called saponification. ORGANOPHOSPHATE ESTERS AND ANHYDRIDES: Esters of phosphoric acids are called phosphate esters. Anions of esters of phosphoric acid, diphosphoric acid, and triphosphoric acid are some of the most widely distributed kinds of substances in living organisms. The main difference between an ester and phosphate ester is, phosphate ester is still a diprotic acid. Its molecules still carry two proton donating OH groups. Each of the two OH groups can be esterified. Nucleic acid molecules, for example, have the phosphate diester system Therefore depending on the pH, a phosphate ester can exist in any one of three forms. There usually is an equilibrium mixture of the three different forms. R/O P OH O O O OH R/ O P OH O- R/O P O- O- At the pH of most body fluids, phosphate esters mostly as the doubly ionized species, a dinegative ion. All forms are usually soluble in water. Diphosphoric acids form diphosphate ester with alcohols. A diphosphate ester has three kinds of functional groups, namely an ester group, three proton donating OH groups, and a phosphoric anhydride system. Adenosine diphosphate, or ADP, is one of many diphosphate esters in the body, existing largely as triply charged anion. ADP reacts with water either to give adenosine and two phosphate ions, or to give adenosine monophosphate and one phosphate ion. Considerable energy per molecule is released in this hydrolysis reaction. The phosphoric anhydride group turns out to be the chief means for storing chemical energy in cells. Since the ADP molecules bear three negative charges, due to the repulsion, they tend to break apart in the presence of a right reactant. But enzyme is required for this hydrolysis reaction in body. The body has no enzymes inside cells that can catalyze this reaction. So energy rich diphosphates can exist in cells despite the abundance of water. Adenosine triphosphate, or ATP, is the most common and widely occurring member of a small family of energy rich triphosphate esters. Because triphosphate esters have two phosphoric anhydride systems in each molecule, on a mole-for-mole basis the triphosphates are among the most energy rich substances in the body. The triphosphates are much more widely used in cells as a source of energy than the diphosphates. enzyme Relaxed muscle + ATP contracted muscle + ADP + Pi Pi = H2PO4- and HPO42Muscular work requires ATP, and if the body’s supply of ATP runs out with no way to replenish, we would rapidly become helpless. The resynthesis of ATP from ADP and Pi is one of the major uses of the chemical energy in the carbohydrates and the fats and oils of our diets. AMINES AND AMIDES: Both amino groups and their protonated form are found in proteins, enzymes and nucleic acids (the chemicals that carry our genes). When a carbonyl group is attached to a N, another family of compounds are formed. They are called amides. They have completely different characteristics compared to amines. Amines are structurally related to amonia, in which one, two or three hydrogen atoms are replaced by alkyl groups. All amines are basic like amonia. Difference between amines and amides: In amine N in –NH2 group is bonded to C of an alkyl group, but in amides N is bonded to C of a carbonyl group C-N bond can’t be broken in amines, but carbonyl-nitrogen bond can be easily broken in amides, provided an appropriate catalyst is present. Naming of amines: The common names of the simple, aliphatic amines are made by writing the name of the alkyl groups attached to nitrogen in front of the word amine (without leaving space). Nitrogen containing, heterocyclic amine rings occur in both proteins and nucleic acids. Those in nucleic acids involve either the pyramidine ring or the purine ring system. N-H group in amine can both donate and accept hydrogen bonds. But they make weaker hydrogen bonds compared to alcohol because N-H bond is less polar than O-H bond. Hydrogen bonds within the molecules of proteins and nucleic acids stabilize their shapes, without which they lose their ability to carry out their biological functions.