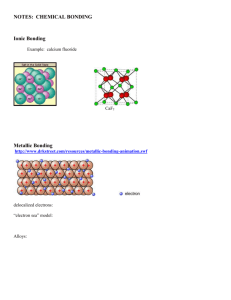
vsepr key - Academics
advertisement

Page 1 V2003 As you know, one can predict the shape and some properties of molecules by using 2 simple rules of thumb: 1) Atoms and lone pairs of electrons take up space 2) In a molecule or ion, atoms and lone pairs orient themselves in space to give everything the most possible room. These simple rules have been codified as Valence Shell Electron Pair Repulsion theory (VSEPR). BEFORE YOU COME TO LAB, READ pp 60-66 in Munowitz on VSEPR. Page 2 V2003 While you are waiting for the IR, complete the following questions for each molecule or ion listed. 1a. Hydronium ion, Give formula: H3O+, Give total # of valence electrons: 8 e-. Its 3-D shape is: tetrahedral (pyramidal) , Its bond angles are: 109.50 , Its bonds are: O H bonds polar, The whole molecule is polar / nonpolar (It’s charged). It looks like: + " O H H " 1. NH3 Give NAME: __ammonia______________________: Give total # of valence electrons: __8____ Its 3-D shape is: __tetrahedral (pyramidal if you ignore lone pairs)________________ Its bond angles are: _ 109.50 ________________ Its bonds are: _Polar/nonpolar (circle one) The whole molecule is polar / nonpolar (Circle one) Is it a weak acid or a weak base (Circle one)? Write the net ionic reaction of NH3 and water. ____NH3 + H2O NH4+ + OHIt looks like: " N H H H Page 3 V2003 2. Ammonium ion Give FORMULA: _____NH4+___________________: Give total # of valence electrons: ____8__ Its 3-D shape is: _______Tetrahedral___________ Its bond angles are: ______ 109.50 ____________ Its bonds are: Polar/nonpolar (circle one)_______polar____________ The whole molecule is polar / nonpolar (circle one)_____polar______________ Is it a weak acid or a weak base (Circle one)? Write the net ionic reaction of NH4+ and water. ____ NH4+ + H2O NH3 + H3O+ It looks like: + H N H H H 3. PO43Give NAME: _____phosphate ion___________________: Give total # of valence electrons: ___32___ Its 3-D shape is: _______tetrahedral___________ Its bond angles are: _____109.5____________ Its bonds are: Polar/nonpolar (circle one)___________________ The whole molecule is polar / nonpolar (circle one)___________________ Phosphoric acid (H3PO4) is a weak acid. Is PO43- a weak acid or a weak base (circle one)? Write the net ionic reaction of PO43- and water. __PO43- + H2O HPO42- + OHIt looks like: 3O P O O O Page 4 V2003 4. Boron trifluoride, Give FORMULA: ____BF3____________________: Give total # of valence electrons: __24____ Its 3-D shape is: ______trigonal planar____________ Its bond angles are: ___120o______________ Its bonds are: Polar/nonpolar (circle one)___________________ The whole molecule is polar / nonpolar (circle one)___________________ Is boron trifluoride a Lewis acid or a Lewis base (circle one)__can accept a pair of e-_________________? It looks like: F F B F 5. AsH3 Give NAME: _____Arsenic trihydride___________________: Give total # of valence electrons: _8_____ Its 3-D shape is: __tetrahedral (pyramidal)________________ Its bond angles are: ___109.5o______________ Its bonds are: Polar/nonpolar (circle one)___________________ The whole molecule is polar / nonpolar (circle one)___________________ It looks like: As H H H Page 5 V2003 6. PF3Cl2 Give total # of valence electrons: _40_____ Its 3-D shape is: _trigonal bipyramidal_________________ Its bond angles are: __90o & 120o_______________ Its bonds are: Polar/nonpolar (circle one)___________________ The whole molecule is polar / nonpolar (circle one)___________________ It looks like: Cl F F P F Cl 7. Bromine trifluoride Give FORMULA: _____BrF3___________________: Give total # of valence electrons: ___28___ Its 3-D shape is: ___ttrigonal bipyramidal/ trigonal if you ignore the lone pairs_______________ Its bond angles are: ___120o______________ Its bonds are: Polar/nonpolar (circle one)___________________ The whole molecule is polar / nonpolar (circle one)___________________ It looks like: " F Br F F " Page 6 V2003 8. XeF4O Give total # of valence electrons: ___42___ Its 3-D shape is: octahedral (square pyramidal if you ignore lone pairs) Its bond angles are: __90o & 120o_______________ Its bonds are: Polar/nonpolar (circle one)___________________ The whole molecule is polar / nonpolar (circle one)___________________ It looks like: O F F Xe F F " 9. SbF6- Give total # of valence electrons: _____48_ Its 3-D shape is: _____octahedral_____________ Its bond angles are: ___120o & 90o______________ Its bonds are: Polar/nonpolar (circle one)___________________ The whole molecule is polar / nonpolar (circle one)__it’s an ion_________________ It looks like: F F F Sb F F F Page 7 V2003 10. ClO2Give NAME: ____chlorite ion____________________: Give total # of valence electrons: _20_____ Its 3-D shape is: __tetrahedral (bent if you don’t count lone pairs)________________ Its bond angles are: _____109.5o____________ Its bonds are: Polar/nonpolar (circle one)___________________ The whole molecule is polar / nonpolar (circle one)___________________ Write the name and formula of the conjugate acid of ClO2-. _____HClO2 chlorous acid_______________ NaClO2 is a weak base. It is soluble in water. Name it: ___sodium hypochlorite_______________ Write the net ionic equation for the reaction of NaClO2 and water. Na+ + ClO2- + H2O Na+ + HClO2 + OH- It looks like: " Cl O : O Page 8 V2003 11. . chloro-trifluoromethane, ClF3C Give total # of valence electrons: __32____ Its 3-D shape is: ____tetrahedral______________ Its bond angles are: ___109.5o______________ Its bonds are: Polar/nonpolar (circle one)___________________ The whole molecule is polar / nonpolar (circle one)___________________ It looks like: Cl C F F F 12. Sulfate ion Give FORMULA: _____SO42-___________________: Give total # of valence electrons: _32_____ Its 3-D shape is: ____tetrahedral______________ Its bond angles are: ______1090___________ Its bonds are: Polar/nonpolar (circle one)________polar___________ The whole molecule is polar / nonpolar (circle one)_____polar (it’s an ion)______________ The name and formula of the conjugate acid of sulfate ion is: ___H 2SO4 sulfuric acid_________________ 2O S O It looks like: 13. Ethylene, H2CCH2, O O Page 9 V2003 Give total # of valence electrons: __12____ Its 3-D shape is: _____trigonal__- trigonal___________ Its bond angles are: ______120o___________ Its bonds are: Polar/nonpolar (circle one)____nonpolar_______________ The whole molecule is polar / nonpolar (circle one)_____nonpolar______________ It looks like: H C C H H H 14. Methyl ammonium ion, CH3NH3+, Give total # of valence electrons: ___14___ Its 3-D shape is: ______tetrahedral - tetrahedral____________ Its bond angles are: _109o________________ Its bonds are: Polar/nonpolar (circle one)__C-H relatively nonpolar, C-N & N-H polar______________ The whole molecule is polar / nonpolar (circle one)________polar___________ Methyl ammonium chloride, CH3NH3Cl, is soluble in water and is a weak acid. Write the net ionic equation for its reaction with NaOH: CH3NH3+ + Cl- + Na+ + OH- CH3NH2 + Cl- + Na+ + H2O It looks like: H H H + C H N H H Page 10 V2003 15, Phosgene, COCl2 (A deadly nerve gas), , Give total # of valence electrons: ___26___ Its 3-D shape is: ____trigonal planar______________ Its bond angles are: ___120o______________ Its bonds are: Polar/nonpolar (circle one)____polar_______________ The whole molecule is polar / nonpolar (circle one)______polar_____________ It looks like: Cl O C Cl 16. CO2, Give NAME: ___carbon dioxide_____________________: Give total # of valence electrons: __24____ Its 3-D shape is: _____linear_____________ Its bond angles are: ___180o______________ Its bonds are: Polar/nonpolar (circle one)___polar________________ The whole molecule is polar / nonpolar (circle one)________nonpolar___________ When CO2 is put in water, it makes a weak acid. Write its formula and name it:____H2CO3 Carbonic acid It looks like: .. .. .. O .. C O .. Page 11 V2003 17. . CH3OH, Methanol SEE THE HINTS ON PUTTING TOGETHER ORGANIC MOLECULES Give total # of valence electrons: __14____ Its 3-D shape is: ___tetrahedral & bent (tetrahedral)_______________ Its bond angles are: __109o_______________ Its bonds are: Polar/nonpolar (circle one)______C-H relatively nonpolar. C-O & O-H polar___________ The whole molecule is polar / nonpolar (circle one)_______polar____________ It looks like: H : : O C H H H 18. CH3CH2 OH, ethanol SEE THE HINTS ON PUTTING TOGETHER ORGANIC MOLECULES Give total # of valence electrons: _20_____ Its 3-D shape is: __tetrahedral-_tetrahedral & bent_______________ Its bond angles are: ___109o______________ Its bonds are: Polar/nonpolar (circle one) __C-C nonpolar, C-H relatively nonpolar. C-O & O-H polar____ The whole molecule is polar / nonpolar (circle one)______polar_____________ It looks like: : H H H O : C C H H H Page 12 V2003 19. CH3 CH2CH2 OH, propanol SEE THE HINTS ON PUTTING TOGETHER ORGANIC MOLECULES Give total # of valence electrons: _26_____ Its 3-D shape is: _tetrahedral-tetrahedral-tetrahedral-bent(Tetrahedral)___________ Its bond angles are: ___109o______________ Its bonds are: Polar/nonpolar (circle one)__C -C nonpolar, C-H relatively nonpolar. C-O & O-H polar____ The whole molecule is polar / nonpolar (circle one)__polar_________________ It looks like: H H H C C H H H H C O : : H