EXEMPT Project Application_Evaluation Form
advertisement

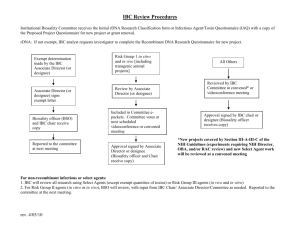
WCHN IBC_Exempt Dealing Application Form/Version B April 09 WOMEN'S AND CHILDREN’S HEALTH NETWORK IBC reference No. Office use only Institutional Biosafety Committee EXEMPT Dealing Project Application and IBC Evaluation Report The WCHN Institutional Biosafety Committee (IBC) must be notified of and approve all work with GMOs which falls under the OGTR Guidelines before it commences, irrespective of whether the work is exempt under Part 1 of Schedule 2 of the guidelines. This form must be completed when Exempt Dealing approval is required. An electronic copy should be emailed to mary.thorne@health.sa.gov.au. An original signed version of this completed form should be forwarded to IBC Executive Officer, Research Secretariat, Level 2, Samuel Way Building, Women's and Children’s Health Network, 72 King William Rd, North Adelaide, South Australia 5006. Queries to Tel: 8161 6390. Section A – Project Details 1 Project Supervisor & Department: (this is the person who will oversee/coordinate the work) 2 Name and Department of other investigators/personnel: 3 Project Title: (this may be the name of the grant application which contains GMO work or it might be a descriptive title of the work performed using GMOs if it is not embodied in a grant). 4 Reason for EXEMPT status: (Applicants should read both Schedules 2 and 3 of the Gene Technology regulations to determine which sort of dealing this is. If after reading these schedules you determine this dealing is exempt, please specify and justify the exemption category. Descriptions of the categories of dealings with GMOs are covered in Schedules 1, 2 and 3 of the Gene Technology Regulations 2001. Specific information relating to Exempt Dealings should also be consulted prior to submitting your application. 5 Brief Description of Project: (provide the names and details of staff to be involved in the project, including training and experience with GMOs, the aim of the project, a brief background to the project, including a description of the GMO and its genetics, and information on risk assessment and risk management). 6 Facilities to be used (include containment level): 7 Commencement date: 8 Certification by Chief Investigator Completion date: Signed: .......................................................... Date: ............................................... Print Name: ................................................................ D:\106747227.doc WCHN IBC_Exempt Dealing Application Form/Version B April 09 Checklist – must be completed by applicant prior to submission. YES Does the title of the project match the grant title? Yes/No (this information is required in order for WCHN to indicate to granting bodies that Biosafety approval has been obtained). If No, please list below, all grant titles that are currently associated with this dealing. Have you consulted the OGTR guidelines to determine whether your application is an EXEMPT dealing? Further information is available at Gene Technology Regulations 2001 (which includes the 2007 amendments) Have you justified this decision in Section A, point 4? Have you provided a brief description of the project in Section A, point 5? Have you made a risk assessment and described your risk management strategy in Section A, point 5? Have you signed the application and kept a hard copy of the application for yourself? D:\106747227.doc WCHN IBC_Exempt Dealing Application Form/Version B April 09 Section B– IBC Assessment (Office use only) 1 (a) (b) (c) (d) The following information has been checked and approved: the aim the details of the personnel involved in the project the description and genetics of the GMO(s) the risk assessment and risk management YES/NO YES/NO YES/NO YES/NO If insufficient information has been provided, return the proposal form to the investigator with a response to provide the required information. 2 The training and experience of the project team for carrying out this type of work are considered adequate 3 IBC Assessment (a) The category of work: EXEMPT [ ] PC1 NLRD [ ] PC2 NLRD [ ] DNIR [ YES/NO ] (b) The physical containment required: PC1 [ ] PC2 [ ] PC3 [ ] OTHER ________________ (c) The type of facility required: Laboratory [ ] Animal House [ ] Plant House [ ] OTHER ________________ (d) Is the proposed facility certified: If yes, please provide the Certification Number: YES/NO Section C– IBC Decision (Office use only) 4 Complete this section for all categories of work: This proposal has been assessed as above and endorsed by the IBC. The following conditions additional to those specified in the guidelines must be adhered to during the conduct of the work: Name of Accredited Organisation: Name of IBC chair: Signature of IBC chair: D:\106747227.doc Women's and Children’s Health Network Date: / /