Current CV/Bio
advertisement

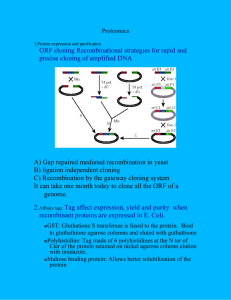
CHRISTIAN TAGWERKER, PH.D. ctagwerk@yahoo.com San Diego, CA 92121 619.727.5320 SCIENTIST / BIOTECHNOLOGIST Research scientist with more than 11 years of experience and expertise in biotechnology, both domestically and internationally. Further expertise: microbial physiology, cloning technology, proteomics, genomics, synthetic biology, biofuels and biopolymers, molecular biology assays and analysis. Demonstrated success in DNA synthesis, test methods development, prototype design and development. Proven ability in laboratory set-up. Recognized for identifying critical issues and implementing effective solutions. Excellent communicator as indicated by breakthrough experiments yielding highly accessed publications in peer-reviewed journals. Core competencies include: • • • • • • • • • • • • • Sequencing/Cloning/Synthesizing Conduct/Design Experiments Bench Production/Scale-ups Test Methods Development /Assays Prototype/Process Design/Building Protocol improvement/Protocol writing Research Publications Understand/Express/Interpret Complex Scientific Information Examine/Data/Objects Lab Set-up Training/Coaching HPLC/GC/GPC/MS Reagent manufacturing/Working knowledge of GMP, ISO, and FDA regulations EDUCATION Ph.D. in Microbiology, Major: Biochemistry, LFU Innsbruck (Leopold Franzens University, Innsbruck) in collaboration as Visiting Scholar with UC Irvine, CA M.Sc. in Microbiology with Honors, Major: Biochemistry, LFU Innsbruck First Diploma in Medicine, and. med. LFU Innsbruck PROFESSIONAL EXPERIENCE University of Basel, Department of Anorganic Chemistry, Basel, Switzerland 2013 – Present POSTDOCTORAL RESEARCHER – HEAD OF BIOLOGY LAB Reporting directly to Head of Department, Professor Thomas R. Ward • Biological applications of artificial metalloenzymes: metabolic engineering of E. coli and Yeast, (S. cerevisiae, Pichia), combined with Streptavidin/Biotin-catalysts for chemical engineering: e.g. in vitro and in vivo (cytoplasm/periplasm) metathesis, C-H activation, imine reduction and hydrogen production. • Defining project work plans, conducted independent research to meet protein (e.g. streptavidin) and chemical (e.g. formate/hydrogen) production standards. • Large scale biofermentations, scale-up to 20 liters from lab bench volumes • High-throughput saturated mutagenesis (HTS) library construction and screening. • Routine BSL1 (Bio Safety Level 1) Lab maintenance, Biosafety and Training, Publication reviews. • • Supervision of 2-3 research technicians and advising undergraduate to masters students throughout the academic year. Presented regular updates for research groups on current and past literature/publications, organized weekly group meetings for 20+ Thomas Ward group members. J. Craig Venter Institute, Synthetic Biology and Bioenergy Group, La Jolla, CA 2008 – 2012 POSTDOCTORAL FELLOW - Reporting directly to Scientific Director and Nobel Prize laureate Hamilton O. Smith and Distinguished Professor Clyde A. Hutchison IIIrd • Analysis of large DNA sequence data sets from Illumina and 454/Roche platforms of bacterial and eukaryotic genomes. • Transplantation of bacterial genomes from one species to another (Mycoplasma) – setup and maintenance of BSL2 (Bio Safety Level 2) laboratory. • Cloning and cultivation of Diatoms and Cyanobacteria with Microbial and Environmental Genomics group headed by Ken Nealson and Andy Allen. • Interaction with various departments within J. Craig Venter Institute – Proteomics and Sequencing facilities, PFGRC (infectious disease department), Plant Genomics, Genomic Medicine, Synthetic Genomics Inc (SGI). • Cloning of intact bacterial genomes in yeast, S. cerevisiae – independently pioneered cloning of an intact cyanobacterial genome (>1.7 Mbases) in yeast, which utilizes the standard genetic code, establishing an important basis for algae cloning platforms in yeast. • Regular research progress reports (3 meetings/teleconferences per week) with group directors and interdisciplinary team of scientists at east coast headquarters, Maryland. • Presentations at scientific conferences and publications in peer-reviewed journals. • Kept concise laboratory notebooks/protocols (requirement to be signed and approved by senior researcher, scanned and submitted in a timely manner). • Regular BSL2 lab Laboratory Safety Training, Injury Illness Prevention Program Training, Fire Safety Training. • Supervision of interns, research associates, new employees, recruiting and annual personnel performance appraisals of existing employees. Department of Physiology and Biophysics, UC Irvine, Irvine, CA 2007 – 2008 POSTDOCTORAL RESEARCH ASSOCIATE – Reporting to Professor Lan Huang • Extension of tandem affinity protein and protein complex purification procedures. • Routine mass spectrometry, sample preparation and analysis, MS data analysis. • Implementation of in vivo protein cross-linking reagents for purification of complex protein mixtures and studying cross-linking reagent fragmentation behavior. • Maintenance and use of QSTAR XL (Applied Biosystems), SQ-D2 (Waters) and Orbitrap (Thermo Scientific) instruments. Vienna Biocenter, Vienna, Austria 2007 POSTDOCTORAL FELLOW – Reporting to Professor Gustav Ammerer • Yeast genetics and cloning • Studied yeast systems biology aspects with a focus on cell signaling. Department of Biological Chemistry, School of Medicine, UC Irvine, Irvine, CA 2002 – 2006 GRADUATE RESEARCH ASSOCIATE – Reporting to Professor Peter Kaiser • Filed disclosure and record of invention at Office of Technology Transfer, UC Irvine, case no. 2005-046-1 – first step toward patent applications. • • • • • • • In vitro synthetic assembly and cloning of a codon-optimized tandem affinity tag (HB-tag) for protein purification under strong denaturing conditions and assessment in S. cerevisiae to study complex ubiquitination and sumoylation profiles. Independently pioneered and optimized an on-bead/resin digestion protocol for optimal peptide generation for subsequent MS (mass spectrometry) analysis. Improved offline fractionation and desalting of peptide samples for LC/LC MS/MS. Construction of HB-tag module plasmid library for rapid PCR-based gene-tagging in S. cerevisiae. Adaptation of the HB-tag method to mammalian cells, mammalian cell line culturing, transfection, DNA/RNA/Protein extraction. Combination of the HB-tag procedure with SILAC (stable isotope labeling of amino acids in cell culture), with or without in vivo cross-linking reagents Identification and publication of novel in vivo ubiquitin chain linkages and ubiquitination sites in S. cerevisiae. Pharmaceutical Factory MONTAVIT GmbH before 2002 QUALITY CONTROL ASSISTANT • Manufacturing processes of prescription medicines for respiratory infections, urinary incontinence and motion sickness. http://www.montavit.com/en/areas-of-therapy • FDA Registration Number: 9680814, GMP-certified facility, ISO 9001:2008, ISO 13485:2007. RESEARCH SKILLS Genetic tools: Primer design, PCR, qPCR, Multiplex, DNA Isolation, Purification, Transformation, Plasmid Construction, Gel Electrophoresis, Southern blotting, Pulsed-Field Gel Electrophoresis (CHEF/FIGE) systems, CsCl gradient ultracentrifugation, Flexstation3. RNA isolation and analysis, Northern blotting. Cloning of whole bacterial genomes into S. cerevisiae. Transplantation of whole genomes from one species to another (i.e. Mycoplasma mycoides LC to M. capricolum). Next generation sequencing platforms: Illumina and 454/Roche, sequencing and analysis. Cell Culture: Biofermentations up to 20 liters (E. coli, Yeast). Mammalian cells, transient transfection in common mammalian cell lines (HeLa), DNA/RNA/Protein extraction. Cyanobacterial culture methods, specifically Prochlorococcus and Synechococcus. Eukaryotic algae culturing techniques: Phaeodactylum tricornutum, Pavlova, Nannochloropsis sp. High-throughput screening (HTS) of E.coli library clones in 96-well format (saturated mutagenesis analysis). Cryopreservation. Proteomics: Protein purifications, 1-D/2-D SDS-PAGE, Western Blotting, affinity chromatography (UPLC, HPLC – GE-AKTA, Waters systems). Mass Spectrometry – sample preparation (in-gel digests, desalting), experience with MALDI, ESI sources, QSTAR XL (Applied Biosystems), SQ-D2 (Waters) and Orbitrap (Thermo Scientific) instruments. Microscopy, Fluorescence microscopy – imaging with Molecular Probes in E.coli, Yeast and eukaryotic algae. Bioinformatics (CLC Genomics Workbench, UNIX scripting, PERL basics, MATLAB, ProteinProspector, Mascot, Analyst, Xcalibur, BioWorks, Igor, Unicorn, Sequin, DNA Strider, pDraw32, Cytoscape, GOnet, GeneBank/Entrez, ChemSketch, MDL ISIS Draw, SoftWare Pro). General Computer Skills for Mac/PC (Illustrator, Word, OpenOffice, Excel, Power-Point, Outlook, Lotus, Photoshop, SmartDraw, Corel, Browsers, EndNote, Mendeley) PROFESSIONAL ACHIEVEMENTS Accepted request to join an inorganic chemistry lab at University of Basel to promote biological applications of artificial metalloenzymes. Performed metabolic engineering of E.coli combined with Streptavidin/Biotin-catalysts and high-throughput screening (HTS) of clones in 96-well plate formats (saturated mutagenesis). Results: Important initial steps for hydrogen overproduction. Identified the urgent requirement for cloning and manipulating intractable, photosynthetic bacteria at a non-profit research institute. Pioneered the first cloning of an intact cyanobacterial genome using the standard genetic code, enabling stable cloning platforms in yeast for algae. Results: Significant scientific breakthrough; important milestone to be met for the J. Craig Venter Institute, Synthetic Biology and Bioenergy group funding. Participated in learning a complex genome transplantation procedure, developed and routinely managed at the headquarters of a research institute. Launched the necessary actions for a biosafety lab (BSL level 2) implementation at a new company location. Results: New work items and media were ordered and transplant clones were obtained in a few weeks. Maintained state of the art practices in culturing microorganisms from bacteria, yeast and sensitive photosynthetic organisms. Carried out required monitoring and sequence analysis by DNA extractions, Results: Breakthrough experiments yielding highly accessed publications in peer-reviewed journals. Focused on protein-protein interactions and modifications during graduate work. Pursued various cloning strategies in budding yeast combined with early DNA assembly methods; initiated combined efforts of a yeast genetics lab and a mass spectrometry group. Results: Protein tagging systems and protein identification methods fostered important new insights related to human cancers and disease. Presented complex data in 25-minute talks during the academic year and at annual departmental retreats for the Biological Chemistry division at UC Irvine. Summarized and designed a presentation based on large data sets from experiments for the entire department. Results: Received an “Outstanding Graduate Student Presentation Award” at the Lake Arrowhead retreat, UCLA Conference Center. Published methods for cloning bacterial genomes in yeast in open access journals. Selected as invited speaker at BIT’s 4th World Genome and DNA Day Conference, Nanjing, China; contributed a presentation summarizing new research data from research group. Results: Received great feedback from attendees; compliments from Senior Scientist at New England Biolabs (NEB), quote: “an excellent talk”. Acknowledged various ethnicities, cultures and ideologies collaborating at academic research institutes. Attended a rigorous schedule of 3 group meetings per week and brought about questions and issues beyond technical and scientific matters from colleagues. Results: Often personal and inter-personal conflicts between different members of the team were resolved and led to a more cohesive unit. Employed at Pharmaceutical Factory Montavit, GmbH as direct employee. FDA Registration Number: 9680814, GMP-certified, ISO 9001:2008, ISO 13485:2007. Results: Acquired knowledge in GMP and ISO regulations and reagent manufacturing processes. Awarded: • • Pre-doctoral 2-year ‘DOC’-Fellowship of the Austrian Academy of Sciences Outstanding Graduate Student Presentation Award, UCLA Conference Center Affiliated with: ASMS, HUPO, ASCINA PUBLICATIONS Karas, B.J., Molparia, B., Jablanovic, J., Hermann, W.J., Lin, Y-C., Dupont, C.L., Tagwerker, C., Yonemoto, I.T., Noskov, V.N., Chuang, R-Y., Allen, A.E., Glass, J.I., Hutchison, C.A. III., Smith, H.O., Venter, J.C., Weyman, P.D. Assembly of eukaryotic algal chromosomes in yeast. Journal of Biological Engineering 7 (1), 1-12 (2013). Tagwerker, C., Dupont, C.L., Karas, B.J., Ma, L., Chuang, R-Y., Benders, G.A., Ramon, A., Novotny, M., Montague, M.G., Venepally, P., Brami, D., Schwartz, A., Andrews-Pfannkoch, C., Gibson, D.G., Glass, J.I., Smith, H.O., Venter, J.C., Hutchison, C.A. III. Sequence analysis of a complete 1.66 Mb Prochlorococcus marinus MED4 genome cloned in yeast. Nucleic acids research 41, 1– 9 (2012). Karas, B.J., Tagwerker, C., Yonemoto, I.T., Hutchison III, C.A. & Smith, H.O. Cloning the Acholeplasma laidlawii PG-8A Genome in Saccharomyces cerevisiae as a Yeast Centromeric Plasmid. ACS Synthetic Biology 1, 22-28 (2012) Guerrero, C., Tagwerker, C., Kaiser, P. & Huang, L. An integrated mass spectrometry-based proteomic approach: quantitative analysis of tandem affinity-purified in vivo cross-linked protein complexes (QTAX) to decipher the 26 S proteasome-interacting network. Molecular & cellular proteomics : MCP 5, 366–78 (2006). Tagwerker, C., Zhang, H., Wang, X., Larsen, L., Lathrop, R.H., Hatfield, G.W., Auer, B., Huang, L. and Kaiser, P. HB-tag Modules for PCR-based Gene tagging and tandem affinity purification in Saccharomyces cerevisiae. Yeast 23, 623–32 (2006) Tagwerker, C., Flick, K., Cui, M., Guerrero, C., Dou, Y., Auer, B., Baldi, P., Huang, L. and Kaiser, P. A tandem-affinity tag for two-step purification under fully denaturing conditions: Application in ubiquitin profiling and protein complex identification combined with in vivo cross-linking. Molecular & cellular proteomics : MCP 5, 737–48 (2006). Kaiser, P., Tagwerker, C. (2005). Is this protein ubiquitinated? Methods Enzymol. 399:243-8. Volunteer Work includes: Basketball-Coach for TI/DSG-Club; Tyrolean Basketball Association (TBV), Innsbruck and Tyrol; Team-Manager of the Herren-Landesliga TI/DSG-Club BasketballTeam (Senior League), Innsbruck, Austria Speaks English (fluent), German (fluent), French Professional References Available Upon Establishment of Mutual Interest