Limestone Pavement Breakdown
advertisement

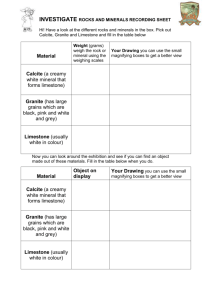
Limestone Pavement Breakdown These are flat areas of limestone blocks resembling paving stones. These stones stand up called clints, and in between each clint (stone) there are grooves (cracks) called grykes. This block appearance is due to 2 key reasons. Firstly, there is the geology & structure of the rock. Limestone is a sedimentary rock, which has many vertical & horizontal cracks in it. The vertical cracks are called joints and the horizontal cracks are called bedding planes. These cracks help to form natural blocks of limestone and allow water to drain through the limestone, meaning it is a permeable rock. A second factor is down to a process called solution (also called carbonation), which is a type of chemical weathering. This process is when carbonic acid runs over the surface of the limestone creating a chemical reaction between the limestone and the acid takes place, which dissolves the rock. This means the clints (blocks) get smaller as their sides are dissolved and so the grykes (cracks) in between get larger. This process gives the pavement a block like appearance. As this takes place there is another change affecting the clint. The carbonic acid running over the top and down the side of the clint dissolves little grooves in the clint. These runnels (grooves) are called Karren. The clint also become rounder over time due to his solution and its hard angular edges are rounded into small clints called Rund Karren. Clints (blocks) Grykes (deep grooves) Rund Karren (rounded blocks) Karren (shallow grooves on clints) The solution takes place down to chemistry. Rain water mixes with carbon dioxide from natural (volcanoes) & human (factories) sources to form carbonic acid also known as acid rain. When it falls on the limestone, (Calcium Carbonate chemical formula CaCO3) it dissolves it to form calcite (called Calcium Hydrogen Carbonate). H2 O Water + CO2 Carbon Dioxide = H2CO3 Carbonic Acid + CaCO3 Calcium Carbonate = H2CO3 Carbonic Acid Ca(HCO3)2 Calcium Hydrogen Carbonate The actual solution of the limestone, actually first takes place when it is covered by soil, thousands of years ago. The carbonic acid develops as rain water drains into the soil and combines with CO2, given off by rotting vegetation. The soil is then often scraped off by a moving glacier exposing the pavement to further solution as we have mentioned already. Limestone Pavement Sheets Appearance Part 1 These are flat areas of limestone blocks resembling paving stones. These stones stand up called clints, and in between each clint (stone) there are grooves (cracks) called grykes. This block appearance is due to 2 key reasons. One reason is the geology & structure of the rock. Limestone is a sedimentary rock, which has many vertical & horizontal cracks in it. The vertical cracks are called joints and the horizontal cracks are called bedding planes. These cracks help to form natural blocks of limestone and allow water to drain through the limestone, meaning it is a permeable rock. Limestone Pavemment Clints (blocks) Grykes (deep grooves) Appearance Part 2 These are flat areas of limestone blocks resembling paving stones. These stones stand up called clints, and in between each clint (stone) there are grooves (cracks) called grykes. This block appearance is due to 2 key reasons. One reason is down to a process called solution (also called carbonation), which is a type of chemical weathering. This process is when carbonic acid runs over the surface of the limestone creating. A chemical reaction between the limestone and the acid takes place, which dissolves the rock. This means the clints (blocks) get smaller as their sides are dissolved and so the grykes (cracks) in between get larger. This process gives the pavement a block like appearance. Limestone Pavement Clints (blocks) Grykes (deep grooves) Appearance Part 3 A process called solution takes place which is when acid rain dissolves the limestone rock which makes up the limestone pavement. When this takes place the carbonic acid runs over the top and down the sides of rocks called clints. The acid then dissolves little grooves (runnels) in the clints (blocks of rocks), which are called Karren. The clints also become rounder over time due to this solution process so its hard angular edges are rounded off into smaller clints called Rund Karren. Limestone Pavement Rund Karren (rounded blocks) Karren (shallow grooves on clints) Chemical Reaction Part 1 Solution, also called carbonation, is a type of chemical weathering affecting limestone. It occurs because of the chemical make up of limestone and results in the limestone being dissolved away. Rain water mixes with carbon dioxide from natural (volcanoes) & human (factories) sources, this forms carbonic acid; also known as acid rain. When it falls on the limestone, (Calcium Carbonate chemical formula CaCO3) it dissolves it to form calcite (called Calcium Hydrogen Carbonate). You’ll need to be able to explain the chemical formula below. H2 O Water + CO2 Carbon Dioxide = H2CO3 Carbonic Acid + CaCO3 Calcium Carbonate = H2CO3 Carbonic Acid Ca(HCO3)2 Calcium Hydrogen Carbonate Acid Rain Calcite Chemical Reaction Part 2 The actual solution of the limestone, actually first takes place when it is covered by soil, thousands of years ago. The carbonic acid develops as rain water drains into the soil and combines with CO2, given off by rotting vegetation. The soil is then often scraped off by a moving glacier exposing the pavement to further solution as we have mentioned already. Pre (Before) Ice Age Soil (CO2 + H2O + Carbonic Acid) Post (After) Ice Age Limestone Blocks Exposed To acid Rain After Soil Removed By Glaciers