Chemical Bonding Unit notes
advertisement

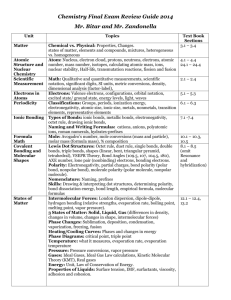
Bonding Notes Bonding - how atoms are held together, determines many aspects of chemical reactivity and physical properties Bonding ideas are based on models - which are simplifications of the way the molecules behave- we must remember that the model gives us a guide for the behavior of atoms, but exceptions do occur- frequently - since the model is intended to simplify reality. I. Types of bonds A. Ionic 1. definition- metal and nonmetal react, both form ions which are attracted to each other - negative and positive ions attract cation - loses electron (+ charge) anion - gains electrons (- charge) 2. Why? - bonds form between metal and nonmetals in this way since the energy of the system is lower than in another form - the ion pair has a lower energy than the separated ions. In general systems will find the lowest energies - they favor states in which their energy is lowest 3. The ions: - group 1 forms +1 ions, group 2 forms +2 ions, group 3 forms +3 ions (loss of electrons - valence shell 8 - stable octet) - group 6 forms -2 ions, group 7 forms -1 ions (gain electrons - valence 8) 4. Transition metals may have > 1 cation formed - review transition metal chemistry - recall all transition metals can be +2 and then others may also form. 5. Ionic bonds are mainly between metals and nonmetals with a large difference in electronegativity values (larger EN difference , greater IONIC character) 6. Solid phase of ionic compound is typically a lattice of ions - packed three dimensionally. The unique stability of ionic compounds refers to the solid state where many ions are held by ionic forces in all directions 7. Lattice energy - the energy released when an ionic solid forms from its ions (this process releases energy and the resulting solid is less energetic and more stable) Lattice enthalpy increases with increasing ionic charge and with decreasing ionic size. So ions that are small and have a bigger charge have a larger lattice enthalpy (take more energy to break apart) than ions that are larger and have a lesser charge (attraction between ions is greater with smaller ions and more charged ions.) 8. ionic compound = a salt - conducts an electric current when in liquid phase (not in solid phase as ions held tight in lattice structure) B. Covalent 1. Bonds form as a result of the tendency to seek the lowest possible energy. 2. Covalent bonds do not have ions - rather electrons are shared between atoms 3. Bonds can be equal sharing of electrons (identical atom or atoms with identical electronegativities: Cl-Cl, C-C, C-S. ) These bonds are NONPOLAR or PURE covalent bonds - each atom shares / needs the electrons equally 4. bonds of unequal sharing result from attraction of atoms with different electronegativities (EN) (unequal desire for electrons) - these bonds are POLAR covalent bonds. The greater the difference in electronegativity between the atoms, the greater the polarity Polar bonds = unequal sharing of electrons C-O (2 non metals → covalent bond) polar bond since EN C=2.5 and EN O = 3.5 O is more desirous of electrons than carbon so those electrons are thought to be found closer to oxygen than carbon - the bond has a more negative side close to oxygen and a more positive side next to carbon we use the symbol delta + (δ+) and delta – (δ -) to show the polarity of bonds and molecules. We also use an arrow with a + side (the point represents the - side) One could say that polar covalent bonds are partially ionic in character although one atom is not seen as taking the electrons and one atom as given the electrons 5. Multiple bonds Covalently bonded molecules can share more than a single pair of electrons: single bond = 1 pair (2 electrons) is shared between adjacent atoms; double bond 2 pairs of electrons (4 e-) are shared between adjacent atoms; triple bond = three pairs of electrons (6 e-) are shared between adjacent atoms 6. Bond length - as more electrons are shared, the length of the bond (distance between atoms) decreases. Triple bond length< double bond length<single bond length 7. Bond strength is a measure of “Bond energy”→ the average energy required to break the bond between 2 atoms. Bond energies (enthalpies) depend not only upon the 2 atoms in question, but also upon the atoms to which those atoms are bonded - therefore average bond energy is used. Table of bond energies is available; units are kJ/mol (higher the bond enthalpy the stronger the bond - more difficult it is to break) a. carbon bond strength: single< double<triple since electrons are held within a smaller area and is more difficult to separate. b. C-O and C=O in carboxylic acid C-O length is longer than C=O (0.143 vs. 0.122) C=O strength is more than C-O (799 vs. 358) ***use bond energies to determine changes in energy for chemical rxn and therefore predict whether the reaction will occur spontaneously or not 8. Electron distribution in molecules see examples: O2, N2, CO2, C2H4, C2H2 - see also Lewis dot C. Metallic bonds 1. Definition: forces that keep metal atoms together 2. Properties of metals a. malleability (sheets), b. ductility (wires), c. good conductors d. high mp 3. Properties lead to the observation that metals are bonded in a strong and nondirectional manner (difficult to separate atoms but easy to move them around) 4. “electron sea model” - metal cations in a sea of valence electrons a. cations are arranged in a lattice- a regular frame in which rows of cations can be moved (hammered, pulled) b. electrons can move to conduct current metal. Cations can be hammered, pulled into wires - non rigid structure c. the electrons do not “belong” to a single cation, but are delocalized and may move about II. Shapes of molecules VSPER theory and Lewis dot A. Lewis dot structures 1. What is it? - shows the arrangement of the valence electrons in an atom - helps identify stable molecules 2. Octet rule - atoms will be most stable energetically if they have a complete octet in their valence (outermost) shell. 3. Electrons can be distinguished as bonding pairs - involved in a bond between atoms, and lone pairs -electrons not involved in bonding. 4. Guidelines for writing lewis dot structures a. total # valence electrons are more important than identifying where the valence electrons come from. b. start with single bonds and progress to multiple bonds c. hydrogen needs only 2 electrons to be complete, all others need eight d. show all electrons (even lone pairs) in structure 5. Exceptions to the octet rule a. boron forms bonds with only 3 pairs of electrons -(BF3) due to small size and large + charge b. > octet SF6 - stable molecule - extra electrons can be placed in the empty d orbitals (so third row and heavier elements MAY exceed octet rule by using empty d orbitals) - examples - ClF3, XeO3, RnCl2, BeCl2 ICl4 B. Resonance - more than one Lewis structure may be written for a compound 1. the “actual” structure is an average of the resonance structures 2. electrons are not always “stuck” between two atoms, but do have the ability to move about a molecule - and act in a delocalized manner. 3. delocalized electrons move around a molecular structure and do not fit our model of Lewis structures (but in nature - true structures are explained this way) examples CO3-2 and benzene (C6H6) C. VSEPR theory - used to predict the shapes of molecules (molecular geometry) 1. the shape of a molecule is important in its reactivity and chemical properties 2. VSEPR (valence shell electron pair repulsion model) - structure is determined by minimizing electron pair repulsions 3. electrons will be arranged as far away from each other as possible (to minimize their interaction (will repulse negative charge) 4. the shapes and bond angles *use models to show shapes a. linear 180 between electron pairs BeCl2 (flat) b. trigonal planar - 120 degrees between 3 pairs (flat) BF3 c. tetrahedral - 109.5 degrees between 4 pairs (3 dimensional) CH4 d. trigonal bipyramid - 90 degrees and 120 degrees - 5 pairs e. octahedral - 90 degrees 6 pairs 5. when the electron pairs are non bonding but lone pairs, the electrons take more space and change the shape of the molecule, for example NH3 3 bonding pairs and 1 lone pair - forms TRIGONAL PYRAMIDAL shape (107 ° rather than 109.5 ° between bonding pairs) H2O 2 bonding and 2 lone pairs 104.5 ° between bonding pairs since lone pairs take up more space than bonding pairs – forms a BENT or ANGULAR shape XeF4 - 4 bonding and 2 lone pairs - square planar molecule ( 90° bond angle) ***lone pairs will occupy positions that have the most space when available in trigonal bipyramid the 120 ° position will be occupied by lone pairs 6. multiple bonds - for determination of structure, multiple bonds count as a single pair 7. molecule polarity determination: polarity of molecule depends on the type and arrangement of bonds. a. when all bonds are identical and evenly spaced (ie. Symmetrical), the molecule is nonpolar b. when bonds are not identical or not evenly spaced, the molecule is polar has a dipole moment III. Intermolecular forces Whereas bonds are intramolecular (within molecules), intermolecular forces are forces between molecules that hold molecules together (not atoms within a molecule). - these forces are weaker than bonds - changes in phase are accompanied by breaking of intermolecular forces A. dipole-dipole attraction 1. Attractive force between polar molecules wherein the positive side of a polar molecule is attracted to the negative side of an adjacent polar molecule 2. The farther apart the molecules, the weaker the force (forces among solid molecules and among liquid molecules would be much stronger that forces in gases - proximity of molecules) B. hydrogen bond (misnomer since not a “true” bond) 1. special type of dipole attraction 2. dipole of high electronegative atoms N,O, F and H atom. H2O experiences H bonds - a stronger dipole attraction (given the high EN of O) NH3 has H bonds HF has H bonds 3. both the high polarity (EN difference) and the small size of H atom(which allows the dipole to be relatively close to the H atom) account for the strength of the force 4. The H bond intermolecular force forms between the lone pair electrons (of the O, F, or N atom in one molecule) and the H in another molecule. So since water has 2 lone pairs in the molecule, it can form 2 H bonds between molecules whereas ammonia, with just 1 lone pair, can form only 1 H bond between molecules. 5. Substances with lone pair electrons (but not HF, HO, or HN bonds) may form H bonds between different molecules, such as carboxylic acids and water molecules (the lone pairs on the carbonyl group may form an IM force with water molecules and hence water solubility is enhanced by a molecules availability to form H bonds C. van der Waal`s forces - forces among nonpolar molecules (aka London dispersion forces) *** note- all molecules have vdW forces but are considered insignificant in polar molecules 1. Instantaneous dipoles can form (since electrons move within their orbitals) 2. This dipole can INDUCE another dipole to form and create a chain rxn of induced dipoles 3. Surface area of molecule determines strength of vdW. Long molecules have greater vdW forces (greater area for separation of induced dipole) than ball shaped molecules. 4. Strength of van der Waal`s forces < dipole < hydrogen bonding D. Intermolecular forces and Boiling Point 1. Boiling Point = temp at which solid → liquid 2. Greater IM forces → higher BP 3. Since forces in H bonds are biggest, those molecules with H bond would be expected to have higher BP than similar molecules without H bonds. 4. van der Waals forces increase with increasing molecular mass ( mass increase → increase in # of electrons → greater chance of instantaneous dipole → greater chance of induced dipoles → stronger intermolecular force) see BP of H2O vs H2S NH3 vs. PH3 C3H8 vs. CH3CHO vs. C2H5OH IV. Physical Properties based on bonding. A. bonding and intermolecular force strength determine the physical properties B. bonding 1.ionic - high mp, non-volatile, conduct as molten and in solution, solubilityrecall rules - many ionic compounds are water soluble 2. covalent- low mp - usually liquid and low melting solids at room temp, nonconductors of electricity, more soluble in nonpolar solvent (mineral oil lab) 3. metals - metallic bonds - mp varies with # of valence electrons; good conductors - all phases (sea of electrons), not soluble in water (may make mixtures of metals - alloys like brass, steel) 4. network solid allotropes of carbon (macromolecules - more later) C. intermolecular forces 1. mp- within group (halogen) increases down table as Molecular Mass increases, vdW forces increase 2. H bond>dipole> van der Waals 3. normally mp determination is a very small range at which solid → liquid 4. with impure substances mp are over a wide range of temperature 5. ionic > polar covalent > nonpolar covalent (pure covalent) 6. bp – liquids → gas - intermolecular forces are key again 7. volatility-ease with which a substance can be changed to a vapor. Those substances with few IM forces (nonpolar) are readily volatile 8. conductivity - allows electric current to flow - need for ions in molten state or aqueous solution ***intermolecular forces have little effect on this as ions or delocalized e- are key 9. solubility - likes dissolve like (polar substances dissolve in water (polar)) a. solubility means the degree to which the solvent molecules surround the solute *** organic structures that can form H-bonds with water are water soluble b. ionic substances can have the polar H2O molecule surround their ions - when very strong forces exist between ions, these substances will not be H2O soluble. If very strong ionic bonds exist, they will not be overcome by water solvation reactions - hence solubility rules c. intermolecular forces and solubility:H bonding > dipole> van der Waals V. Hybridization A. another model of bonding and structure B. hybridization - mixing of atomic orbital to form new orbitals to hold bonding electrons - bonding usually only considers valence electrons - consider CH4: C = 1s22s22p2 and H = 1s1 with our model of bonding and structure the valence e- in C are 2s2 2p2 then H will bond with 2s2 electrons and 2p2 electrons which would imply that two different types of bonds will be formed; HCs and HCp. All information about methane leads to the conclusion that all the CH bonds are equivalent. -- to explain this phenomenon we consider that the orbitals that electrons occupy in covalent bonding situations are hybridized orbitals. ---hybridized orbitals are equivalent orbitals which originate as different orbitals. --- 1s and 3p atomic orbitals combine to form 4 sp3 hybrid orbitals which have identical energies and differ only in their orientation in space _ _ _ 2p _ _ _ _ sp3 hybrid _ 2s C. tetrahedral shapes are sp3 hybridized orbital - 4 bonding or non bonding pairs = sp3 hybrid D. sp2 hybrids can also exist 1. sp2 hybrids are 3 equivalent bonding orbitals _ _ _ 2p _ _ _ sp2 _2p _2s 2. CH2CH2 uses 1 s orbital and 2 of 3 of the p orbitals to form 3 equivalent hybrid orbitals called sp2. 3. CH2CH2 has a double bond between the carbon atoms. The double bond can be considered a combination of two bonds: i. the sigma bond - is the bond in the plane between the two carbon atoms (the hybridized orbital is a sigma bond) ii. the pi bond - is the bond above and below the planes of the carbon atoms a. two parallel bonds above and below the C-C plane b. the non hybridized 2p atomic orbital E. sp hybrids 1. 2 equivalent bonding sp orbitals composed of 2s and a single 2p orbital 2. in carbon that leaves 2 unhybridized 2p atomic orbitals; those unhybridized orbitals can form 2 pi bonds 3. each pi bond will form 2 parallel areas above and below plane of C-C sigma the different pi bonds will differ in their orientation in space F. work with hybrid orbital and shapes of molecules 1. sp3 = tetrahedral 2. sp2=trigonal planar 3. sp = linear VI. Delocalization of electrons A. delocalization - means that electrons are not held by a single atom or pair of atoms the electrons are smeared over an area of space and not attached to an individual atom B. Benzene’s structure is best described by delocalization of the pi bonded electrons the sigma bonds keep electrons in the plane between two atoms, pi bonds are electrons above and below the plane between the atoms. Bonds between carbons are all equivalent 1.5 bonds rather than single or double bonds between carbons. This supports the resonance structures discussed earlier in that the resonance diagrams indicate an average of those structures. VII. Structures of Allotropes of carbon A. Allotropes of carbon: different forms of elemental carbon 1. diamond 2. graphite 3. fullerene B. Network solid - describes the bonding among atoms - Giant molecule - “network” of atoms to form a huge molecule - rather than discrete individual molecules the whole network is assumed. C. Diamond -hardest naturally occurring substance -electrical insulator (does not allow current to flow) -each carbon is surrounded by a tetrahedral structure of other carbon atoms -sp3 hybridization -directional covalent bonding between c atoms in tetrahedral shape in large network -uses: due to hardness - cutting implements D. Graphite -slippery , black , conductor -carbons are bonded in layers of rings -sp2 hybridization - each C has 3 other carbons in a trigonal planar structure -additional electrons occupy pi bonds in planes above and below -delocalized pi electrons allow electric current to be conducted in graphite lack of bonding between layers allows graphite layers to slide past one another E. fullerenes C60 In the proposed structure for C60, each vertex of the truncated icosahedron (big soccer Ball – see p. 440 Chang)) is occupied by a carbon atom, and each carbon is threeconnected to other carbon atoms by one double bond and two single bonds. Carbon atoms with this kind of connectivity are sp2 - normally planar but fullerenes are NOT planar as high symmetry distributes ring strain across the entire structure