GST Protein Purification

GST Protein Purification
1.
Inoculate an E.coli
colony containing the transformed plasmid into 5 ml LB media with 100µg/ml ampicillin; grow at 37 C overnight at 250 rpm.
2.
Inoculate 100 mL LB/Amp (1:20 dilution of O/N culture) in 500 ml flask. Grow
1-2 hours at 30 C. Higher dilutions can be used for time convenience.
3.
Check OD600. Want final OD between 0.5 and 1.0
4.
Add 0.5M IPTG to 0.5 mM concentration to induce fusion protein expression, and incubate culture for a further 2-3 hours.
5.
Pour cells into two 50 ml blue top tubes and pellet at 4.000 rpm for 10 min, 4 C in centrifuge; discard media. [at this point cell pellets can be stored at -20 C for later use].
6.
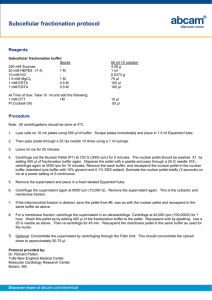
Resuspend each cell pellet in 10 mL GST binding buffer 20 mMNaHPO4 pH 7.5,
150 mM NaCl, 3 mM KCl, 1 mM EDTA (+ protease inhibitors). Keep cells on ice.
7.
Lyse cells with cell disruptor.
8.
Spin down cell debris at 15,000 rpm for 20 minutes at 4 C in Oakridge tubes (35 mL polypropylene) and SS-34 rotor; use high-speed centrifuge. Make sure tubes are balanced.
9.
Transfer sup. to new 50 ml tube. R etain a 20µl sample of pooled supernatant for
SDS-PAGE analysis; also freeze pellet for later gel check. Filter before loading onto GST column
Purification of a GST fusion protein
10. The supernatant was filtered (0.2 µm filter) before applying to GSTrap.
11.
GSTrap columns were equilibrated with 4 column volumes (CV) PBS (pH 7.3) and the lysates were applied (8 ml on GSTrap 1 ml and 40 ml on GSTrap 5 ml). The columns were washed with 10 CV PBS and eluted using 5 CV 50 mM
TrisCl (pH 8.0) 10 mM reduced glutathione (Fig 2). The runs were completed in 25 min using ÄKTAexplorer 10.
12.
(
Elution buffer = 10mM reduced glutathione/ 50mM Tris-HCL pH 8.0/ 5% glycerol ).
Grose, 2010