Tables
advertisement

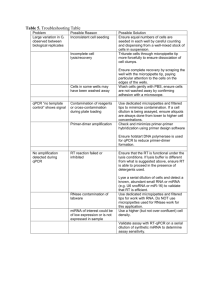
Table 1. Lysis Buffer Composition Component Volume per sample (µl) Nuclease Free Water 5x ImProm-II Buffer 15 10 10% Triton X-100/Nonidet P40 8 dNTPs (10 mM) MgCl2 (25 mM) RNase-Free DNase 2.5 2.5 2 Total Volume 40 Comment The final RT volume will eventually be 50 µl (see below) The final concentration of Triton X-100 and Nonidet P40 is 2% each. The enzymatic activities of DNase and reverse transcriptase are not affected under these conditions. Table 2. RT Master Mix Component Volume per sample (µl) Nuclease Free Water ImProm-II Reverse Transcriptase RT Primers (1 µM each) 2.5 2.5 Comment 5 The final concentration of each RT primer is 100 nM. Total Volume 10 The total volume for RT is 50 µl. Table 3. qPCR Master Mix Component Volume per sample (µl) Nuclease Free Water 2x SsoFast EvaGreen Supermix Forward qPCR Primer (10 µM) 3.4 10 Reverse qPCR Primer (10 µM) Diluted cDNA product 0.8 5 Total Volume 20 0.8 Comment Primer concentrations may have to be titered for optimal performance according to the qPCR reagents used Table 4. qPCR Thermal Cycling Parameters Description Temperature (oC) Time (s) Cycles Enzyme activation / Initial denaturation Denaturation Annealing/Extension 95 30 1 95 60 5 30 50 Table 5. Troubleshooting Table Problem Large variation in Ct observed between biological replicates Possible Reason Inconsistent cell seeding Incomplete cell lysis/recovery Cells in some wells may have been washed away qPCR “no template control” shows signal Contamination of reagents or cross-contamination during plate loading Primer-dimer amplification Possible Solution Ensure equal numbers of cells are seeded in each well by careful counting and dispensing from a well-mixed stock of cells in suspension. Triturate cells through micropipette tip more forcefully to ensure dissociation of cell clumps. Ensure complete recovery by scraping the well with the micropipette tip, paying particular attention to the cells on the edges of the wells. Wash cells gently with PBS, ensure cells are not washed away by confirming adhesion with a microscope. Use dedicated micropipettes and filtered tips to minimize contamination. If a cell dilution is being assayed, ensure aliquots are always done from lower to higher cell concentrations. Check and minimize primer-primer hybridization using primer design software Ensure hotstart DNA polymerase is used for qPCR to reduce primer-dimer formation. No amplification detected during qPCR RT reaction failed or inhibited RNase contamination of labware miRNA of interest could be of low expression or is not expressed in sample Ensure that the RT is functional under the lysis conditions. If lysis buffer is different from what is suggested above, ensure RT is able to proceed in the presence of detergents used. Lyse a serial dilution of cells and detect a known, abundant small RNA or miRNA (e.g. U6 snoRNA or miR-16) to validate that RT is efficient. Use dedicated micropipettes and filtered tips for work with RNA. Do NOT use micropipettes used for RNase work for this application. Use a higher (but not over-confluent) cell density. Validate assay with RT-qPCR on a serial dilution of synthetic miRNA to determine assay sensitivity.