UKMi Q&A xx - NHS Evidence Search
advertisement

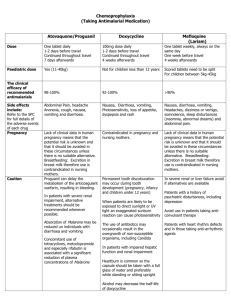
Medicines Q&As Q&A 162.2 How should nausea and vomiting be treated during pregnancy? Prepared by UK Medicines Information (UKMi) pharmacists for NHS healthcare professionals Before using this Q&A, read the disclaimer at www.ukmi.nhs.uk/activities/medicinesQAs/default.asp Published: August 2012 Summary Most cases of nausea and vomiting in pregnancy resolve within 16-20 weeks with no harm to the pregnancy (1). Prescribing treatment in the first trimester is usually not indicated unless the symptoms are severe and debilitating (1). NICE recommend that non pharmacological treatments should be tried first, such as changes in diet, use of ginger or wrist (P6) acupressure (1). No drug is specifically licensed for the treatment of nausea and vomiting in pregnancy though some anti-emetics are considered compatible with pregnancy. First line recommended treatment is an antihistamine such as promethazine or cyclizine, both of which can cause sedation. Second line treatments are prochlorperazine and metoclopramide. Background Nausea is the most common gastrointestinal complaint in pregnancy, occurring in 80-85% of all pregnancies, with vomiting an associated complaint in approximately 52% (1). Nausea and vomiting during pregnancy is mainly due to human chorionic gonadotrophin (HCG). Symptoms usually begin at around 6-8 weeks, peaking at about 9 weeks, when HCG levels are at their highest, and often settling by 20 weeks (1;2). Although it is often referred to as ‘morning sickness’, nausea and vomiting can occur any time of the day. Only 11-18% of affected women have sickness confined to just the mornings (1). Nausea and vomiting do not have harmful effects on the pregnancy but the pregnant woman’s quality of life can be severely affected, with day-to-day activities hampered, greater use of healthcare resources and time off work (1). Hyperemesis gravidarum affects less than 1% of all pregnancies (2). It refers to pregnant women in whom fluid and electrolyte disturbances or nutritional deficiency develops from intractable vomiting early in pregnancy (1). This Q&A does not discuss the treatment of hyperemesis gravidarum. Answer Both non-pharmacological and pharmacological measures are used to treat nausea and vomiting of pregnancy (NVP). Non-pharmacological measures should be tried first. If pharmacological treatment for nausea and vomiting during pregnancy is used, the drugs should be taken regularly to achieve adequate blood concentrations. If oral preparations cannot be tolerated, then other administration routes can be used (3). The different drugs can be taken in combination. There is limited evidence regarding the safety and efficacy of most drug therapies, including anti-emetics, during pregnancy, but available studies and extensive clinical experience support the efficacy and rationale for using treatment options that are available and used in clinical practice (4). Drugs may be prescribed when the potential benefit justifies the potential foetal risk, without evidence of harm (4). Medicines Q&A 34 has advice on what needs to be considered when prescribing to pregnant women. Non-pharmacological measures There are a number of non-pharmacological measures that may help initially, though there is little published evidence regarding the efficacy of dietary changes (2): Dry, bland food From the NHS Evidence website www.evidence.nhs.uk 1 Medicines Q&As Eat little and often Ensure good hydration Avoid high fatty foods Ginger has been used for reducing the severity of nausea and vomiting but there are conflicting data, which may be due to different preparations used.(2). One randomised controlled study (n=70 up to week 17 gestation) showed a significant improvement in nausea score (rated on a visual analogue scale) with ginger 1g/day when compared with vitamin B6 40mg/day for 4 days, but the average number of vomiting episodes did not differ between the two groups.(5) The number of subjects and duration of therapy was too short to assess effects of ginger on pregnancy outcome. There is a lack of clear safety data and doses of ginger higher than those usually found in a normal diet should be avoided (3). However, no adverse effects on the foetus are expected (1). A number of randomised controlled trials have studied the efficacy of P6 (wrist) acupressure in pregnancy. All but one of the studies showed a positive effect for stimulation of the P6 pressure point and a reduction in nausea and vomiting; the remaining study showed no difference between acupressure and no treatment. No difference in adverse foetal outcomes (perinatal outcome, congenital abnormalities, pregnancy complications) were seen between acupressure, sham acupressure and no treatment (1). Conflicting data regarding the efficacy of pyridoxine (vitamin B6) for nausea and vomiting during pregnancy come from two randomised, controlled studies(4). One study found that a dose of 25mg three times a day did not improve mild-to-moderate symptoms but did improve severe nausea and reduced the number of vomiting episodes, while the second study showed that 30mg/day reduced the number of vomiting episodes (4). A retrospective cohort study of pyridoxine in pregnancy (n= 458 treatment group, n=911 control group) and case-controlled / cohort studies in 170,000 pregnant women taking a combination anti-emetic preparation containing pyridoxine 10mg (up to 40mg/day could be taken for severe symptoms), did not show an increase in adverse effects on the foetus (4). It is worth noting that the UK Committee of Toxicity of Foods recommends that the maximum safe amount of pyridoxine to take per day is 10mg and that NICE do not currently recommend pyridoxine to treat nausea and vomiting in pregnancy (1). Drug treatment Antihistamines, such as promethazine, cyclizine and prochlorperazine, and metoclopramide, are used to treat nausea and vomiting in pregnancy with no conclusive evidence of an increased risk of congenital abnormalities above the background rate for the population (1;3;6). Metoclopramide can cause extrapyramidal reactions in younger women (15-19 year olds) (6). Domperidone is not recommended due to the lack of observational or trial data (7;8) although it is known to be used in clinical practice. There is less evidence on the safety and efficacy of 5HT 3 inhibitors such as ondansetron (9). The choice of drug will be based on its adverse effect profile as well as its safety in pregnancy. Factors such as patient preference and preparations available may also play a part when choosing a drug. Table 1 provides an overview of the preparations available and standard dosing regimens of the antiemetics discussed in this Q&A. Antihistamines indirectly affect the vestibular system and decrease stimulation of the vomiting centre (4). In a meta-analysis of 24 trials from 1960-91 involving more than 200,000 women given antihistamines (specifically H1-blockers) during the first trimester, no increase in teratogenic risk was seen (4). This low risk of anti-emetics has been reinforced by a more recent Swedish study of 665,572 births registered over a 7 year period in which 29,807 women reported the use of antiemetics during their pregnancy (10). The overall malformation rate from all infants was 4.7%, with the rate amongst those exposed to anti-emetics being 4.3%. The weakness with the study is that antiemetic use may be under-reported, and there was little information on the duration and amount of drug use (10). The drawback of using first generation antihistamines is that they cause sedation, but the fact that they have been used for a long time with no evidence of adverse effects on the newborn is reassuring (11). From the NHS Evidence website www.evidence.nhs.uk 2 Medicines Q&As First-line pharmacological agents Promethazine and cyclizine Short-term treatment with an antihistamine such as promethazine or cyclizine is recommended as first line treatment (6). The Collaborative Perinatal Project identified 114 pregnancies that were exposed to promethazine during the first trimester and 746 pregnancies that included the use of promethazine anytime during gestation. No evidence suggesting an increased risk of malformations associated with promethazine use was found in either group (8). A study in 165 babies exposed in the first trimester also failed to show an association between promethazine use and malformations (8). In a surveillance study, a total of 61 birth defects occurred in 1197 infants exposed to promethazine during the first trimester (51 expected); more cardiovascular defects occurred than were expected (n=17/12), but other factors such as the mothers disease, concurrent drug use and chance may have been involved (8). In a large population-based case-control study (22,843 cases with malformations and 38,251 controls), no difference in the frequency of promethazine use was seen between the two groups (12;13). Animal studies have reported teratogenicity with cyclizine but data from human studies have not indicated an increase in malformations after foetal cyclizine exposure. The studies include data on 111 mothers given cyclizine during the first trimester, a small cohort in the Collaborative Perinatal Project and retrospective studies, that did not find a significant association between first trimester exposure to antihistamines (including cyclizine) and malformations (8). No association between oral clefts and cyclizine exposure in the first trimester was found in a study of 599 children (8). Second-line pharmacological agents Prochlorperazine and metoclopramide Prochlorperazine, (a phenothiazine), and metoclopramide, (a dopamine receptor blocker), are also considered safe to use during pregnancy as a short term measure, but are reserved for second line treatment as less data are available (3;6). Although case reports of children with malformations born to women treated with prochlorperazine during the first trimester include cleft palates, congenital heart defects and limb abnormalities, but these observations do not implicate prochlorperazine as the causative factor since more recent, robust studies have not replicated these findings.(14) Observational studies in 704 pregnancies (Michigan Medicaid) and 877 pregnancies (Collaborative Perinatal Project, CPP) with first trimester exposure did not find any statistically significant increase in birth defects and do not support an association between prochlorperazine and congenital defects (8). For use any time during pregnancy, 2023 exposures were recorded in the CPP but again, no evidence was found to suggest a relationship to malformations or an effect on birth weight, perinatal mortality or IQ scores at 4 years (8). Metoclopramide has been used at all stages of pregnancy without evidence of embryo- or fetotoxicity in human (and animal) studies (8). In a study of 126 women who took metoclopramide in the first trimester, data from a Scandinavian Prescription database of 309 women and a US surveillance study in which 192 newborns had been exposed to metoclopramide during the first trimester, the rates of spontaneous abortions, preterm deliveries and congenital abnormalities were no different to those expected in the general population (8). A large retrospective cohort study (3458 singleton pregnancies exposed to metoclopramide during the first trimester) showed that metoclopramide use was not associated with an increased risk of minor congenital malformations, multiple malformations or clusters of anomalies, or an increased risk of pre-term birth, low Apgar scores or perinatal death (15). The investigators did not assess whether metoclopramide caused extrapyramidal side effects in the mothers. Data from a prospective study (n=175 received metoclopramide, with paired, matched controls) conflicts with that above, showing a higher rate of premature births (8.1%) compared with controls (2.4%), but rates of major malformations did not differ between the two groups (3;16). As stated above, metoclopramide can cause extrapyramidal side effects, particularly in young women, such as dystonic-dyskinetic head and neck movements. There are limited reports of this From the NHS Evidence website www.evidence.nhs.uk 3 Medicines Q&As occurring in women taking metoclopramide during pregnancy. A retrospective chart review of 301 women using subcutaneous metoclopramide reported extrapyramidal symptoms in 11 women (17). Ondansetron The scant evidence regarding the use of ondansetron during pregnancy does not suggest that it will increase the risk of congenital abnormalities but it may be advisable to use a drug with more experience in pregnancy. There are a few case reports of ondansetron use during pregnancy, from weeks 11-13, weeks 30-33 and weeks 14 to 33. In all three cases, all babies delivered were healthy (8). A retrospective case analysis described the use of ondansetron for hyperemesis gravidarum in 16 pregnancies (18). Ondansetron was used, on average, at 11.8 weeks gestation, but in seven cases was used during the period of organogenesis (4th to end of 10th week). No major congenital anomalies that could be attributed to ondansetron were seen; a heart murmur with ventricular and atrial septal defects was seen in one baby whose mother had been treated with ondansetron from week 23 (6th day). Two babies were born prematurely; one born at 24 weeks died due to complications of prematurity, the second was born at 36 weeks and had a lower birth weight. Three babies had intra-uterine growth retardation, from under-nutrition of the mothers because of persistent nausea and vomiting. A population-based study, using data from registries and hospital records, compared the outcomes of 251 pregnancies exposed to ondansetron (263 children, 211 exposed during first trimester) with controls (number not stated) (19). Ondansetron use was not associated with an increased risk of major birth defects (Odds ratio (OR) 1.1, 95% CI 0.6 to 2.0), but newborns were more likely to have a lower birth weight and birth length, and there was an increase in pre-term delivery (OR 1.8, 95% CI 1.2 – 2.5). Analysis of this study is limited by the fact that data have come from a conference abstract only. Manufacturer’s recommendations The Summary of Product Characteristics (SPC) of the anti-emetics discussed in this Q&A state a variety of cautions for the use of the drugs during pregnancy. The drug manufacturers state that the use of promethazine and prochlorperazine is not advised in pregnancy unless the physician considers it essential.(20-24) Metoclopramide should only be used when there are compelling reasons and not in the first trimester (25;26). Domperidone should only be used when justified by the anticipated therapeutic benefits due to limited post-marketing data.(7;27) As some animal studies suggest that cyclizine may be teratogenic, the manufacturers of cyclizine do not recommend its use due to the lack of human data (28;29). Animal studies of ondansetron do not indicate either direct or indirect effects on the embryo and because its use has not yet been established, the manufacturers of ondansetron do not recommend its use in pregnancy (30-32). Routes of administration: Table 1: Drugs available for treating nausea and vomiting Drug Licensed indications Dose Preparations available (6) Cyclizine (28;29) Nausea and vomiting. Orally or by IM or slow IV injection: 50mg up to three times a day Tablets, injection Nausea and vomiting. Orally: 10mg up to three times a day Rectally: 60mg twice a day Tablets, liquid, suppositories. Nausea and vomiting. Orally: 10-20mg 2 - 3 times a day IM injection: 25-50mg, max 100mg/day Tablets, liquid, injection Severe nausea and vomiting. Oral tablets: 5mg three times a day , max 30mg/day Buccal tablets: 3-6mg twice a day. IM: 12.5mg stat followed by oral medication 6 hours later Tablets, buccal tablets, liquid, intramuscular injection Domperidone (7;27) Promethazine hydrochloride (20;22) Prochlorperazine (21;23;24) From the NHS Evidence website www.evidence.nhs.uk 4 Medicines Q&As Metoclopramide (25;26) Nausea and vomiting. Ondansetron (3032) Nausea and vomiting associated with chemotherapy, radiotherapy and post-operative. Dose in adults >20 years of age and young adults 15-19 years ≥60kg: Orally or by IM or slow IV injection: 10mg up to three times a day Dose in adults >20 years of age and young adults 15-19 years 30-59kg: Orally or by IM or slow IV injection: 5mg three times a day Note that doses for ondansetron given here are based on those used for chemotherapy and are for guidance only. Orally: up to 8mg twice a day Rectally: 16mg/day IV or IM: 8mg up to 12 hourly Tablets, liquid, injection Tablets, orodispersible tablets, liquid, injection, suppositories. IM: intramuscular injection IV: intravenous injection Consult each SPC for full prescribing details. Limitations This Q&A does not discuss the treatment of hyperemesis gravidarum, a disorder in 0.3-2% of pregnant women in whom fluid and electrolyte disturbances or nutritional deficiency from intractable vomiting develops in early pregnancy (1). This condition usually requires hospital admission for intravenous fluids, electrolyte replacement and sometimes nutritional support (2). This Q&A does not discuss the use of combinations of anti-emetics or of drugs used that are not normally part of the treatment pathways, such as nabilone or haloperidol. References (1) Antenatal care: routine care for the healthy pregnant woman. Clinical Guideline March 2008. National Collaborating Centre for Women's and Children's Health www.nice.org.uk (2) Jarvis S, Nelson-Piercy C. Management of nausea and vomiting in pregnancy. Br Med J 2011; 342:doi: 10.1136/bmj.d3606. (3) Treatment of nausea and vomiting in pregnancy. March 2010. UK Teratology Information Service Accessed via www.toxbase.org on 03/05/2012. (4) Badell ML, Ramin SM, Smith JA. Treatment options for nausea and vomiting during pregnancy. Pharmacotherapy 2006; 26(9):1273-1287. (5) Ensiyeh J, Sakineh M-AC. Comparing ginger and vitamin B6 for the treatment of nausea and vomiting in pregnancy: a randomised controlled trial. Midwifery 2009; 25:649-653. (6) British National Formulary 63rd edition. March 2012. Ryan, RSM. editor. British Medical Association and Royal Pharmaceutical Society of Great Britain. http://www.bnf.org/bnf/ (7) Summary of Product Characteristics. Motilium 10. Date of revision of the text: 28/05/10. Accessed 04/05/12. McNeil Products Ltd. www.emc.medicines.org.uk. (8) Briggs GG, Freeman RK, Yaffe.S.J. Drugs in pregnancy and lactation. Ninth edition. Philadelphia.: Wolters Kluwer / Lippincott Williams & Wilkins., 2011. (9) American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Nausea and vomiting of pregnancy. Obstet Gynecol 2004; 103(4):803-815. From the NHS Evidence website www.evidence.nhs.uk 5 Medicines Q&As (10) Asker C, Norstedt Wikner B, Kallen B. Use of antiemetic drugs during pregnancy in Sweden. Eur J Clin Pharmacol 2005; 61:899-906. (11) Lee A. Common problems in pregnancy. In: Lee A, Inch S, Finnigan D, editors. Therapeutics in Pregnancy and Lactation. Oxon: Radcliffe Medical Press Ltd, 2000: 23-36. (12) Use of promethazine in pregnancy. October 2010. UK Teratology Information Service Accessed via www.toxbase.org on 03/05/2012. (13) Bártfai Z, Kocsis J, Puhó EH et al. A population-based case-control teratologic study of promethazine use during pregnancy. Reprod Toxicol 2008; 2008(25):2-276. (14) Treatment of nausea and vomiting in pregnancy. March 2010. UK Teratology Information Service Accessed via www.toxbase.org on 03/05/2012. (15) Matok I, Gorodischer R, Koren G et al. The safety of metoclopramide use in the first trimester of pregnancy. N Engl J Med 2009; 360(24):2528-2535. (16) Berkovitch M, Mazzota P, Greenberg R et al. Metoclopramide for nausea and vomiting of pregnancy: a prospective, multicenter international study. Amer J Perinatol 2002; 19(6):311316. (17) Reichmann JP, Kirkbride MS. Reviewing the evidence for using continuous subcutaneous metoclopramide and ondansetron to treat nausea & vomiting during pregnancy. Manag Care 2012; May:44-47. (18) Ferreira E, Gillet M, Lelièvre J et al. Ondansetron use during pregnancy: a case series. J Popul Ther Clin Pharmacol 2012; 19(1):e1-e10. (19) Colvin L, Slack-Smith L, Stanley F et al. Pregnancy outcomes in women dispensed ondansetron in pregnancy. [Conference abstract]. Birth Defects Research part A - Clinical and Molecular Teratology 2012; 94(5):404. (20) Summary of Product Characteristics. Phenergan 25mg tablets. Date of revision of the text: 08 September 2011. Accessed 04/05/12. Sanofi-Aventis www.emc.medicines.org.uk. (21) Summary of Product Characteristics. Prochlorperazine tablets BP 5mg. Date of revision of the text: 30/1/12. Accessed 04/05/12. Actavis UK Ltd. www.emc.medicines.org.uk. (22) Summary of Product Characteristics. Phenergan injection. Date of revision of the text: 05 October 2011. Accessed 15/08/12. Sanofi-Aventis www.emc.medicines.org.uk. (23) Summary of Product Characteristics. Prochlorperazine injections BP 12.5mg/mL, 1mL & 2mL. Date of revision of the text: 30/11/11. Accessed 15/08/12. Mercury Pharma Group. www.emc.medicines.org.uk. (24) Summary of Product Characteristics. Buccastem 3mg. Date of revision of the text: December 2011. Alliance Pharmaceuticals. www.emc.medicines.org.uk. (25) Summary of Product Characteristics. Maxolon tablets 10mg. Date of revision of the text: January 2011. Accessed 04/05/12. Amdipharm Plc. www.emc.medicines.org.uk. (26) Summary of Product Characteristics. Maxolon injection. Date of revision of the text: January 2011. Accessed 08/15/12. Amdipharm Plc. www.emc.medicines.org.uk. (27) Summary of Product Characteristics. Motilium suppositories 30mg. Date of revision of the text: 30/03/11. Accessed15/08/12. Zentiva. www.emc.medicines.org.uk. From the NHS Evidence website www.evidence.nhs.uk 6 Medicines Q&As (28) Summary of Product Characteristics. Valoid tablets. Date of revision of the text: October 2009. Accessed 04/05/12. Amdipharm Plc. www.emc.medicines.org.uk. (29) Summary of Product Characteristics. Valoid injection. Date of revision of the text: October 2009. Accessed 15/08/12. Amdipharm Plc. www.emc.medicines.org.uk. (30) Summary of Product Characteristics. Zofran suppositories. Date of revision of the text: 17 February 2012. Accessed 15/08/12. GlaxoSmithKline UK. www.emc.medicines.org.uk. (31) Summary of Product Characteristics. Zofran tablets 4mg. Date of revision of the text: 18 May 2012. GlaxoSmithKline UK www.emc.medicines.org.uk. (32) Summary of Product Characteristics. Zofran injection, Flexi-amp injection. Date of revision of the text: 1 August 2012. Accessed 15/08/12. GlaxoSmithKline UK. www.emc.medicines.org.uk. Quality Assurance Prepared by Alexandra Denby, Pharmacist, London Medicines Information Service (Northwick Park Hospital) Date Prepared August 2012 Checked by Sheena Vithlani, Pharmacist, London Medicines Information Service (Northwick Park Hospital) Date of check September 2012 Search strategy Embase: PREGNANCY AND [VOMITING OR NAUSEA] [Limit to: Publication Year 2010Current and Human and English Language Embase: VOMITING/dt [dt=Drug Therapy] OR NAUSEA/dt [dt=Drug Therapy] [Limit to: Publication Year 2010-Current and Human and English Language] Embase: [PROCHLORPERAZINE OR PROMETHAZINE OR METOCLOPRAMIDE OR ONDANSETRON OR CYCLIZINE] AND PREGNANCY AND [VOMITING OR NAUSEA] [Limit to: Publication Year 2010-Current and Human and English Language] Medline: PREGNANCY AND NAUSEA/OR VOMITING Medline: PREGNANCY AND [PROCHLORPERAZINE OR PROMETHAZINE OR METOCLOPRAMIDE OR ONDANSETRON] In-house database/ resources Clinical experts: Sobia Mohammad, Lead Pharmacist for Womens and Children and Miss Mandish Dhanjal, Consultant Obstetrician, Imperial College Healthcare NHS Trust, Hammersmith Hospital From the NHS Evidence website www.evidence.nhs.uk 7
![Questionnaire used in the study Demographics GENDER: M [ ] F](http://s3.studylib.net/store/data/006712173_1-21c851410b04058d524e1b79e54e32b0-300x300.png)



![[Physician Letterhead] [Select Today`s Date] . [Name of Health](http://s3.studylib.net/store/data/006995683_1-fc7d457c4956a00b3a5595efa89b67b0-300x300.png)
