ПРАВИЛА ОФОРМЛЕНИЯ ТЕЗИСОВ
advertisement

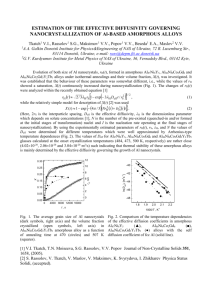
BULK PROPERTIES OF THE Cu50Zr50 ALLOY V. Bykov, D. Yagodin, T. Kulikova, I. Sipatov, K. Shunyaev The Institute of Metallurgy of Ural Division of Russian Academy of Science, Russia k_shun@mail.ru One of the most extensive and thriving research lines of modern material science is investigation of bulk amorphous metal alloys. Among ensemble of such alloys the special attention is given to the systems based on Cu-Zr. This system has a unique glass forming ability: the CuZr alloy is one of the few binary metal systems, which is succeeded to form the bulk-amorphous samples. Furthermore, these samples are possessed excellent mechanical properties, corrosion-resistance and high values of thermal stability. Small quantity addition of other metals (Al, Ni, Ti, rare earth metals, etc.) results in improvement of glass-forming ability as well as application of additives enables to obtain bulkamorphous alloys with critical thickness, which is sufficient for industrial application. Since obtaining the first bulk amorphous samples in 2004 [1], research boom of the Cu-Zr system has been started. The general mystery is the nature of glass-forming ability of the Cu-Zr system as well as relations between the ability and system composition and elements additive concentrations. It was experimentally determined the bulk amorphization regions are concentrated in a very narrow composition ranges (less than 1 at %), which are not correlated with eutectic or other characteristic points at phase diagrams. All efforts to explain such performance has been failed. However it is necessary to note the range of experimental techniques and theoretical approaches, which are applied as a rule for the task, is rather limited. With reference to what has been noted above about the Cu-Zr alloys, the main reason of interest is due to their glass-forming ability. Therefore a number of physicochemical properties of alloys and intermetallics based on Cu-Zr are little-studied in crystal state at high temperatures. There are no detailed data about thermal properties behaviour (thermal conductivity, heat diffusivity and capacity, density, etc.) for the Cu-Zr alloys when rising temperature. In the present work the density of the equiatomic Cu-Zr alloy were studied by absolute gamma-radiation technique using the unit described in the literature [2] and NETZSCH 402CD dilatometer using 237 highly sensitive sensor-transducer of linear displacement. Dilatometry tests were performed at constant rates of heating and cooling 2 K/min in an high purity helium atmosphere. Investigated sample was synthesised in an induction furnace Density determination tests were performed by gamma-radiation densimeter in a protective pure helium atmosphere at constant rates of heating and cooling 2 K/min in 23 mm diameter graphite crucibles. After fusion the liquid sample was thoroughly blended by a special mechanical stirrer. The stirrer is tube of beryllium oxide which was inserted into protective graphite cover withtungsten-rhenium thermocouple. To ensure compositional uniformity and absence of gaseous pores a gamma-quantum intensity was measured along vertical axis starting from the melt surface (~8 mm) and down to the crucible bottom. The beam of gamma-quantum were passed through crucible at the 2 mm distance from its bottom. The thermocouple in protective cover was inserted into the melt. The cover was placed slightly higher the examined zone. At the exposure time of 1000 s the counting rate of gamma-quanta passed through the sample was in the range of 1700 1800 s-1 that corresponds to the random error of 0.15 % at the accuracy 0.95. The existence of the two phase’s composite-mechanical mixture obtained by inductive remelting has been determined on evidence derived from X-ray diffraction and microstructure analysis. The phases are amorphous and crystalline. Measurement results obtained by dilatometer in several heating cooling cycles of temperature dependence of density for the Cu 50Zr50 in solid state are shown in Fig. 1. As may be inferred from Fig 1, the sample was not fully crystalline even after 3 times heating up to 850oС and followed by cooling. The observed crystallization and amorphization processes cycling for low heating and cooling rates is characteristic feature of bulkamorphous composites based on the Cu-Zr system. The problem was scrutinized in the studies [3, 4]. The temperatures T1-3 corresponds to the following transformations: T1 = 265oС – the initiation of martensite transformation during first heating (As). The determined temperature value is in agreement with experimental results [5], where As equals 255oС for the 238 Cu50Zr50 alloy, T2 = 540oС – the amorphous phase crystallization; T3 = 726oС – the eutectoid transformation CuZr ↔ Cu10Zr7 +CuZr2. Fig. 1 The relation between density and temperature for the Cu50Zr50 alloy obtained by dilatometer. Measurement results for temperature dependence of density in Cu50Zr50 sample for solid and liquid states obtained on gammadensimeter are shown in Fig. 2. For crystal state (5th heating in Fig.1) the density dependence of temperature is described by the equation (in the range of T = 25726 oС): dS(T) = 7.34 – 2.27·10-4∙T –2.90·10-4∙T2 (1) In liquid state density tends to decrease linearly with the increase of temperature, this dependence is described by the equation dL(t) = 7.49 – 5.43·10-4∙T 239 (2) Fig. 2 Temperature dependence of density in the Cu50Zr50 sample obtained by gamma-densimeter The difference between density values of composite sample (data of the 1st heating) and crystalline one (data of the 5th heating) was determined as 0.4% at room temperature. The density-temperature dependence (ρcr – ρcom)/ρcom is shown in Fig. 3, which demonstrates the density distinctions of composite and crystalline samples. The gamma-radiation technique and dilatometry were applied to density measurement of the equiatomic CuZr alloy in the temperature range from room one and up to 1200°С, which correspond to solid and liquid states. Parameters for linear approximation of density-temperature dependences were calculated from the density values which were found experimentally. Furthermore, the density differences between initial composite sample and completely crystalline were determined. 240 Fig. 3 Density differences between composite and crystalline samples The cycling of crystallization and amorphization processes was found out during heating and cooling of the sample obtained by induction technique. Acknowledgements This work was supported by Russian Scientific Foundation (grant RNF 14-13-00676). 1. 2. 3. 4. 5. References D. Xu, B. Lohwongwatana, G. Duan, et. al. Acta Materialia 52, (2004) 2621. G. Sivkov, D. Yagodin, P. Popel. High Temperature 44, (2006) 535. T. Nagase, A. Nino, T. Hosokawa, Y. Umakoshi. Mat. Trans. 48, (2007) 1651. T. Nagase, Y. Umakoshi. Scripta Materialia 48, (2003) 1237. C. Biffi, A. Figini, A. Tuissi. Intermetallics 46, (2014) 4. 241