Kori Bustard Husbandry
advertisement

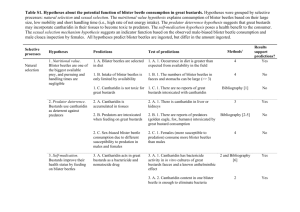
Kori Bustard Husbandry Sara Hallager, Smithsonian National Zoological Park 3001 Connecticut Ave., Washington DC 20008 Introduction The bustards comprise the Family Otididae in the Order Gruiformes. Bustards are medium to large-sized terrestrial birds, chiefly inhabiting open plains in either arid or seasonally dry regions of the old world. The bustard family is made up of 25 species in 11 genera and most are in need of conservation. Two species are listed by the IUCN (International Union for Conservation of Nature and Natural Resources) Red List of Threatened Animals as Endangered (great Indian bustard Ardeotis nigriceps and lesser florican Sypheotides indicus). The Bengal florican (Houbaropsis bengalensis) is listed as Critically Endangered. Two species are listed as vulnerable (great bustard Otis tarda and houbara bustard Chlamydotis undulata) and six more are listed as near-threatened (Australian bustard Ardeotis australis, blue bustard Eupodotis caerulescens, little brown bustard Eupodotis humilis, Denham’s bustard Neotis denhami, Nubian bustard Neotis nuba and little bustard Tetrax tetrax). Several species of bustard are so poorly known that their true conservation status cannot be determined. Agricultural changes, overgrazing, hunting, trapping, habitat loss, droughts and wars are the main threats to bustards (del Hoyo et al., 1996). Bustards are completely terrestrial, and are strong, but reluctant, fliers. Bustards vary widely in weight from the adult male kori bustard (Ardeotis kori) at 19 kg to as little as 0.55 kg in the little bustard (Tetrax tetrax). Bustards prefer open habitats. On the ground, they are nervous and alert, and move into cover at the first sign of danger. When foraging, bustards are slow walkers, taking invertebrates, small vertebrates, and vegetable matter from scrub or grassland. Bustards do not have a crop, but consume large quantities of grit, have a powerful gizzard, and have a highly developed cecum to aid in digestion. The mating system is still unclear for many bustard species. They have variously been described as probably monogamous or polygynous or promiscuous although present evidence indicates the majority are polygynous (Johnsgard, 1991). The largest bustard, the kori, is indigenous to the grasslands and lightly wooded savannas of southern and eastern Africa. The nominate subspecies Ardeotis kori kori occurs in Botswana, Zimbabwe, Namibia, southern Angola, South Africa and Mozambique (Johnsgard, 1991), while Ardeotis kori struthiunculus occurs in Ethiopia, Kenya and Tanzania. The species is listed in Appendix II of CITES, and the South African Red Data Book lists the status of A. k. kori as Vulnerable (Brooke, 1984). According to Collar (1996), the kori bustard is showing signs of chronic decline and local extinction over its entire range. Causes for this decline include increasing agriculture and development, hunting pressure, and a low tolerance for human activity (Dale, 1990). Reduced breeding activity in dry years compounds the problem (T. Osborne, pers. comm.). Kori bustards were first reported in US zoos in 1940 (Hallager, 2007) but did not begin breeding until 1992 when Dallas Zoo hatched the first bird. Since that time 111 birds have been born at seven facilities. Kori bustards have been managed at the Species Survival Plan (SSP) level since 2000. Two other Photo by Jessie Cohen bustard species are maintained in US zoos including the buffcrested bustard (Eupodotis rufricrista gindiana) (managed as a PMP (Population Management Plan)) and white-bellied bustard (Eupodotis senegalensis) (managed at the DERP level (Display, Education, Research Population)). Management strategies for breeding vary according to the species. White-bellied bustards are monogamous and adults are maintained in pairs. Buff-crested bustards tend to be bred in captivity as pairs but have been managed in groups of multiple females and males. Kori bustards are best managed as trios. Successful propagation of bustards depends on a thorough knowledge of bustard natural history and careful attention and dedication to the species sensitive needs. By nature, all bustards are shy and wary. Historically, insensitivity to this trait prevented breeding, limited life spans and earned them a reputation of being hard to keep. Wild-caught birds are shy of humans, as are parent-reared captive offspring. Hand reared birds are not as nervous around humans. The larger bustards are easily startled by people, noises, quick movements, ungulates and feral animals. Large adult bustards are susceptible to a variety of disorders similar to those found in ratites. Most of these problems are associated with improper handling and management (Bailey et al., 1997). Advances in bustard husbandry over the past 20 years have led to an understanding of the special needs of bustards that make caring for them different than other birds. This paper outlines some of the husbandry needed to successfully manage kori bustards. Enclosure size Pen size must be large enough for kori bustards to escape keeper intrusion. The suggested minimum pen size is 13 m x 20 m per bird, and birds will be much better off if provided with a few hectares of space. The preferred boundary for large bustard enclosures is one-inch chain link mesh. This size mesh reduces the chances of chicks getting out, large rodents getting in and any chance of a bird getting a leg caught in the fence during a capture. Even though some birds are successfully maintained in exhibits with a 2.4 m high fence the preferable height is 3 - 3.7 m. Kori bustards are powerful flyers and even flight-restrained birds can escape a 2.4 m fence when startled or on a windy day. Site barriers, such as thick shrubs placed 1.3 - 2.5 m in from the perimeter walls, give the birds a sense of security in pens of minimum size (Hallager and Boylan, 2004). Native, feral predators are dangerous for both adult and young bustards. Foxes and raccoons have attacked adult kori bustards, sometimes fatally, and small chicks left on exhibit usually disappear. Thus, enclosures must be built to minimize predator access. Digging predators (e.g. dogs, foxes) can be excluded by burying the base of the boundary 0.3 m in the ground. Surrounding the enclosure with electrical wire can deter climbing predators. Covered pens are necessary if hens are allowed to raise chicks. In areas where large predators (e.g. coyote, bobcat and cougar) are common, birds may need to be housed indoors at night although this practice will inhibit breeding. Facilities in zones where temperatures fall below 0°C must have winter holding facilities available. Bustards are susceptible to frost bite and must not be left outside during periods of freezing rain or snow or when temperatures are below freezing. Even facilities that do not experience cold weather should have a shelter available. The shelter can be used for times when pen repairs are needed, for medical confinement, to minimize food loss from wild birds or when birds must be caught. Kori bustards do not thrive in climates that are consistently wet, rainy and damp. These conditions lead to poor feather condition and unhealthy birds. Likewise, exhibits that offer no areas of sun are also detrimental to bustard health. Kori bustards need areas of full sun to allow them to dry off damp feathers. Additionally, areas of shade should be available, especially in hotter climates. Capture and transport Kori bustards, especially adult males, are very powerful birds and require somewhat different handling skills from other long-legged birds of similar size. Bustards are highly sensitive to stress and incorrect handling can cause temporary or permanent neural damage, hyperthermia, fractures of legs or wings, skin lacerations, bruising and feather loss, luxation of the tibiotarsal bones, dislocation of the cervical vertebrae, compression of the trachea and internal organs, capture myopathy and even death (Siegel et. al., 2007). Nets should not be used with koris as the risk of injury escalates greatly with this capture technique. Two people are needed to capture and handle a bird and, in the case of males, it is absolutely essential to avoid injuries to the bird. Adult males require that a second person be there to take additional control of the legs and wings. A person of small stature (male or female) may be incapable of adequately restraining an adult male kori, regardless of their skill and experience with birds. The capture is preferably done in a small, darkened and enclosed area such as a shed with solid walls so that the chance of evasion is greatly reduced. Whenever avoidable, do not corner a bird against chainlink fence as the birds are more likely to receive trauma to their beaks, heads, carpal joints and feet. If birds are to be captured in their yard, make certain that the fence line at the capture area is at least 2.13 m high, preferably more. Even pinioned koris can jump high. The ease of capture will vary widely, depending on exhibit design. If the birds are accustomed to coming into a shed or stall daily for their food, there may be less stress in the capture because the birds can be enticed in with food. Once in the containment area it is very important to catch the bird quickly to minimize stress and chance of injury. The preferred method of restraint is to tuck the body of the bird under one arm with the head at the handlers back. The weight of the bird rests primarily on the holder's forearm while the other hand is used to restrain the legs. Legs are generally not tucked up under the body, as it is possible for a bird to break its leg if is too tightly restrained this way when it struggles. Restrain the legs at the tarsal joint with at least one finger keeping the legs apart so that they do not abrade the joints against each other. Once in hand, do not to apply pressure to the body from above to make the bird “sit”; as this may cause the bird to resist as well as put undo pressure on the legs. It is not necessary to restrain the head (koris do not poke like cranes), however, it may be in the best interest of the restrainer to have the head under control. Hooding can calm birds but hand reared birds may prefer to remain un-hooded. For internal transfers it is better if the bird can be hand-carried as this will reduce the problems associated with recapture once the bird is released from its crate. Feeding Kori bustards are omnivorous, consuming mostly insect and plant material in their grassland habitat. Small vertebrates are consumed when available. The gastrointestinal tract is typical of an insectivorous bird (Maloiy et al., 1987). The esophagus is not as pronounced as that of a carnivorous bird and the ventriculus is thick and muscular (a trait characteristic of birds consuming complex food items such as insects and plant material; Stevens and Hume, 1995, Klasing, 1998). Koris have a pronounced cecum, which is common in omnivorous birds such as ostriches, rheas, cranes, and quail (Klasing, 1998). Although koris are clearly not carnivores, they have been fed in captivity as “primarily carnivorous” omnivores. The kori bustard SSP is working to develop a more omnivorous diet and the current recommended diet in is based on nutritionally complete feeds, whole prey (vertebrate and invertebrate), and produce. Recommended crude protein in diets offered to captive koris should range between 16.530.0% on dry matter basis. Based on the reported foraging strategy of free ranging kori bustards, proposed diet proportion guidelines are presented in Table 1. These guidelines assist with diet formulation by proportion in order to insure that nutrient needs are met (and levels of specific nutrients are not grossly exceeded, i.e. protein). When ingredients are combined according to the proportions recommended diets can be formulated to meet the proposed nutrient guidelines for koris (Maslanka and Ward, 2004). Kori bustards should be fed twice a day, with additional feedings to allow for management and behavioral needs. Diet can be offered in pans or tubs or hand-fed to individual birds in a group. Kori bustards will consume pelleted foods (e.g. crane pellets), so pellet dispensers should be placed in pens to encourage the consumption of dry, nutritionally complete feeds. Kori bustards require only small areas of water from which to drink and fresh, clean water should be available at all times. They are not heavy drinkers but do drink on a daily basis. Heated water dispensers for northern zones are recommended. Bustards do not bathe in water (they dust bathe) so pools are not needed in exhibits (except for aesthetic reasons). If pools are present in enclosures, they should be shallow enough that a bird can walk through the water and the sides should gradually slope to the deepest portion. Pools deeper then 0.6 m are not recommended. Table 1. Kori bustard recommended diet proportion guidelines (as fed basis) (Kori Bustard Husbandry Manual. Hallager and Boylan, 2004). Item Minimum, Percent of Diet Maximum, Percent of Diet Vertebrate Prey 0 25 Invertebrate Prey 5 30 Nutritionally Complete Feeds* 40 55** Produce 10 20 * Nutritionally complete feeds are those designed to meet specific recommended nutrient levels. ** Diets which exceed 55% complete feeds can be considered. A diet comprised of 75% complete feed has maintained captive kori bustards (Anderson 1995). Medical concerns Although bustards do not have many unique infectious disease conditions, a wide variety of diseases have been reported in both wild and captive species (Bailey, in press). Although generally hardy birds, most large bustards in captivity die as a result of complications from puncture wounds or compound fracture of legs or wings in addition to complications caused when housing with incompatible exhibit mates. Nervous kori bustards will run into or pace in contact with their perimeter fence or pen walls. It is important to use fencing materials that are smooth to avoid abrasions to faces or pinion sites. Koris may compact the soil by chronic pacing, and may become prone to lameness and pododermatitis (Siegel et.al. 2007). Kori bustards in zoo settings need to be carefully monitored for signs of impaction and zinc toxicity as they have a particular tendency to consume items thrown into their enclosures (e.g. pennies, camera batteries, nails, etc) by visitors. Daily inspection of pens and the removal of foreign material are very important. Significant viral diseases of bustards include Newcastle disease, avian pox and avian influenza. In the Middle East trichomonosis is probably the most important protozoal disease of captive bustards. (Silvanose et. al., 1997). It has been documented several times in the US in kori bustards. Kori bustards should be screened biannually for parasites and de-wormed if necessary. Anthelmintic and antiprotozoal medication can be given in the water or food when needed (Bailey & Hallager, 2003). Vaccination policies for all species depend upon individual institution policies that are generally based upon a risk versus benefit analysis. This analysis usually involves the prevalence of the specific disease, subsequent threat of exposure, efficacy and safety of a vaccine, and the risk to the bird. Studies evaluating the susceptibility of koris to West Nile virus (WNV) have not yet been conducted, and given the relatively low incidence of reported morbidity and mortality of WNV in koris, it may not be necessary to vaccinate this species. Hemosiderosis has been reported in some captive kori bustards, but the cause of this is not yet known. Some theories include either a possible dietary etiology or perhaps a genetic predisposition (S. Murray, personal communication 2007). Mixed species exhibits For optimal breeding and management, kori bustards are best housed by themselves. Although some species have been housed with kori bustards successfully, before integrating other species with koris, it is recommended that institutions with successful mixed-species exhibits be contacted to determine specific exhibit parameters. The SSP Coordinator should also be contacted for advice on compatibility. For zoos with small enclosures, it is recommended that breeding flocks of kori bustards be housed by themselves. Zoos with larger enclosures may be able to house their koris with other suitable species of birds and mammals, although even zoos with large pens may opt to house kori bustards by themselves to encourage reproduction. Table 2 can assist managers in their selection of compatible species. It is not a complete representation of compatibility, as this will be determined to some extent by the specific behavior of individuals and the physical conditions at each institution. Full details regarding kori bustards and mixed species exhibits can be found in the “Kori Bustard Species Survival Plan (Ardeotis kori) Husbandry Manual” (Hallager and Boylan, 2004). It is strongly recommended that kori bustards not be housed with species that are known to show aggressive behaviors. Table 2. List of species interacting successfully or aggressively with kori bustards (Kori Bustard Husbandry Manual, Hallager and Boylan 2004). Species where aggressive Species successfully housed with Kori bustards. encounters have occurred Bird Mammal Bird Hooded vulture Blesbok East-African crowned Crane (probably Egret Dik-dik most crane species are incompatible) Guinea fowl Duiker Ostrich Flamingo Gazelle sp Marabou stork Ground hornbill Impala Saddle-billed stork (probably most Sacred ibis Kudu stork species are incompatible) Mammal Secretary bird Nyala Waterfowl Giraffe* Giraffe* Egyptian vulture Pygmy hippo Demoiselle crane Springbok European white stork Topi Lappet-faced vulture Waterbuck African spoonbill Zebra Gerenuk *Kori bustards have been killed by giraffe Social organization Captive female kori bustards develop a social hierarchy among themselves, and subordinate females may suffer attacks about the face and head. If given sufficient room (approximately 250 m 2 per bird) and a few well-placed thick shrubs which can act as visual barriers, females may be housed together. Males should be housed separately from each other. Dominance interactions come in both subtle and overt forms. In captivity, dominant males are known to attack and even kill subordinate males (Siegel et.al. 2007). Reproduction In the wild, male kori bustards become sexually mature between 4-6 years, and females between 3-4 years, although this may be reduced in captivity (Hallager and Boylan, 2004). Breeding season varies throughout the US, with breeding commencing as early as February in southern zones and ending as late as October in northern zones. Reproductively active males and females can become more aggressive to both conspecifics and keepers during the breeding season. At the onset of the breeding season, male kori bustards begin to display to attract females. Early in the season, the display is infrequent and of low intensity. At the start of the breeding season, males begin to gain weight. At the National Zoological Park, males increase in body mass by as much as 4 kg over a nine week period (Hallager, 2005). Testosterone levels also rise as weight increases (Hallager, 2007). During this time, displays intensify and aggression (i.e. chasing) towards females escalate. Once started, male reproductive displays reach their maximum several weeks after the initial onset and continue through the breeding season. Male kori bustards engage in partial display in which they cock their tails, partially inflate their esophagus and strut around. As full display nears, the esophagus inflates to as much as four times its normal size and resembles a balloon. With neck expanded, the tail and wing feathers pointed Photo by Jessie Cohen downward, and the crest erected, the male emits a low-pitched six-note booming vocalization as he snaps his bill open and shut (Astley-Maberley, 1937, Hoesch, 1938, Hellmich, 1988), occasionally throwing his head backwards in an attempt to make himself more visible and establish territory (Hallager, 2007). Females choose the time of fertilization. Copulation lasts only a few seconds, and is performed on the ground. It is preceded by up to 20 minutes of head pecking by the male to the recumbent female. Females often lose head feathers during this processes. Once over, the male leaves to resume displaying. He plays no part in incubation or chick rearing. At the end of the breeding season, males lose weight rapidly. A weight loss of 3 kg is not uncommon over a 3 week period and is accompanied by molt, a drop in testosterone and a complete cessation of breeding activity (Hallager, 2007). Females typically begin laying eggs 4-6 weeks after males have begun to display. In southern zones, laying occurs as early as February while in northern zones, females lay starting in May (Hallager and Boylan 2004). The average clutch size in US zoos is 1.4 eggs (Hallager. 2007). Females will lay replacement clutches if previous clutches are pulled. A female entering reproductive condition creates a nest scrape, usually placed next to a shrub or large rock for camouflage. The 1-2 pale to dark olive eggs with brownish markings are laid at 2 day intervals. Some females begin incubation with the first egg, while others begin after the arrival of the second egg. Egg dimensions in kori bustards in the US average 82 mm x 57 mm and average weight is 149 grams (Hallager, 2007). Incubation is 22-24 days. The female alone incubates the eggs, leaving occasionally to eat. She is very aggressive towards keepers and other birds that approach her nest. Bustard chicks begin vocalizations at internal pip. Hatch occurs about 48 hours after internal pip, 12-24 hours after external pip. Chicks are precocial and nidifugous at birth. Following birth, chicks are usually dry within an hour or two. Eyes are open upon hatch, although they have an opaque appearance to them for the first several days. Chicks are somewhat mobile approximately four to six hours after hatch. They are not fully mobile until they are 24 hours old. Artificial Incubation and Rearing Eggs should be moved to an incubator for safe keeping as soon as they are laid. Dummy eggs can be placed under the female to keep her sitting. Incubators should be set to 37.50C with a relative humidity of 50-60%. Target weight loss for kori bustard eggs is 14.9% (Anderson, 1998). Eggs are turned every two to four hours. The thickness and pigmentation of the egg makes candling difficult so fertility is often determined by observing the egg seven days prior to hatch and watching for movement. At internal pip, move the egg to a hatcher unit set to 37.40C and increase the relative humidity to 75%. Do not turn the egg at this point. Hatching should occur within 24 hours after external pip. Hand-rearing is the recommended method for raising kori bustards and produces birds tolerant of human disturbance and handling. Future reproduction has been shown not to be compromised in kori bustards. Details of hand-rearing kori bustards can be found in the AZA kori bustard husbandry manual (Hallager and Boylan, 2004). References Anderson, S. 1998. Captive management and breeding of the kori bustard (Ardeotis kori) at the National Avian Research Center. National Avian Research Center, Abu Dhabi, External Report. Astley-Maberly, CT. 1937. Notes on birds from North-Eastern Transvaal. Ostrich 8: 10-13. Bailey, T., Naldo, J., Samour, J. and Howlett, J. 1997. Bustard Therapeutics: Techniques for the medical management and care of captive bustards. External Report No. 5, Wildlife Veterinary Research Institute, Environmental Research and Wildlife Development Agency, PO Box 4553, Abu Dhabi, United Arab Emirates. Brooke, R.K. 1984. South African Red Data Book: Birds. CSIR, Pretoria. Collar, N. J. 1996. Family Otididae (bustards). Pp.240-273 in del Hoyo, J., Elliott, AD and Sargatal, J. eds. Handbook of the birds of the world vol. 3. Hoatzin to auks. Lynx Edicions, Barcelona, Spain. Dale, J. 1990. The Kori bustard in central Zimbabwe. Honeyguide 36(3): 123-128. Del Hoyo, J., Elliot ,A. & Sargatal, J. eds 1996. Handbook of the Birds of the World. Vol. 3. Hoatzin to Auks. Lynx Edicions, Barcelona. Hallager S. and Boylan J. 2004. Kori bustard SSP husbandry manual. Washington, D.C. Smithsonian National Zoological Park. Hallager, S. 2007. International Studbook for Kori bustards. Smithsonian National Zoological Park. Hallager, S. 2007. New Display Behavior in Male Kori Bustard (Ardeotis kori struthiunculus). The Wilson Journal of Ornithology 119(4): 750-755. Hellmich, J. 1988. Zum Balz-Verhalten der Riesentrappe (Ardeotis kori). Zoologische Garten 58: 345-352. Hoesch, W. 1938. Zur Balz von Choriotis kori. Ornithologische Monatsberichte 46:110-113. Johnsgard, P. 1991. Bustards, Hemipodes, and Sandgrouse: Birds of Dry Places. Oxford University Press. Klasing, K. 1998. Comparative Avian Nutrition. CAB international, New York, NY. Maloiy, G., Warui, C. and Clemens, E.. 1987. Comparative Gastrointestinal Morphology of the Kori bustard and Secretary Bird. Zoo Biol. 6:243-251. Maslanka, M. and Ward, A. 2004. In Hallager S. and Boylan J. 2004. Kori bustard SSP husbandry manual. Washington, D.C. Smithsonian National Zoological Park. Siegel, C., Hallager, S. and Bailey, T. 2007. Bustards. In: G. Holland (ed.), Encyclopedia of Aviculture: Volume I. Hancock House Publishers. Stevens and Hume 1995. Comparative Physiology of the Vertebrate Digestive System. Cambridge University Press, New York, NY