Title: Investigating Enzyme Activity
advertisement

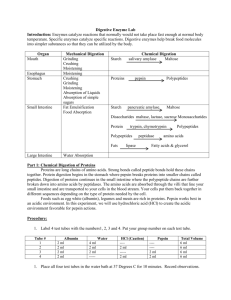
Title: Investigating Enzyme Activity Aim: To investigate the effect of temperature on enzymatic rate of reaction Enzyme molecular structure is affected by temperature. To assess the effect of temperature on enzyme activity, or reaction rate, the following experiment is carried out. In this experiment samples of amylase – a starch digesting enzyme – are exposed to various temperatures for 5 minutes. The time required for each sample to digest the same quantity of starch at the same temperature is then estimated by using iodine solution (which turns blue black in the presence of starch) as an indicator. Try to make the method as quantitative as possible so that you can say how much faster the reaction occurs at one temperature than another. Requirements Test tube rack (2) Test tubes (10) Labels Stopclock Syringes (10cm³) White tile Glass rods Water baths maintained at 25°C, 40°C, 60°C and 100°C Thermometers Beakers Amylase solution Starch solution Iodine solution Ice Procedure 1. Label 5 test tubes as follows: - Room temperature (measure it!), 25°C, 40°C, 60°C and 100°C 2. Add 5cm³ of amylase solution to each test tube. 3. Label an additional 5 test tubes as follows: S room temperature (measure it!), S25°C, S40°C, S60°C and S100°C. Add 5cm³ of starch to these test tubes. You should now have a total of 10 test tubes in front you, 5 with amylase solution and 5 with starch solution. 4. Keep the two room temperature test tubes at room temperature, and place the other test tubes into the appropriate water baths for exactly 5 minutes. 5. During this time, prepare a results sheet/ table with time as the first column, and an additional 5 columns for each of the temperatures. Lower Six Biology – 2008 Make sure the starch and amylase solutions both reach the temperature of the water baths they were in before you mix them. 6. Starting with your test tubes at the lowest temperature, add the 5cm³ of starch solution to the appropriately labelled tube with amylase solution and mix the resulting solutions with a clean glass rod. 7. At intervals of 30 seconds (starting at 0 seconds), test the mixture for the presence of starch by withdrawing one drop of the starchenzyme mixture, place it on a white tile, and then add one drop of iodine solution. 8. Repeat steps 6 and 7 for each temperature. Take care to use a separate glass rod for each tube and a different one for the iodine solution. 9. Carefully record each result as it is obtained in terms of a consistent colour scheme. Do not continue your observations for longer than 10 minutes (600 seconds). 10. In a separate (2nd) summary table record how long it took for the blue-black colour to cease to be obtained when the iodine solution was added to each reaction mixture, and convert this time to a rate. (Note: you will have to decide what colour represents the end point of the reaction). 11. Plot a graph of the time taken to reach the endpoint (as a rate) versus temperature, and describe it. For Consideration: 1. What are the components of starch and how do they react with iodine in potassium iodide solution? 2. When starch is broken down by amylase, what are the end point products? 3. In your experiment what was the colour of the endpoint of the reaction that you observed? 4. Suggest how you might relate the colour of the iodine to the proportion of starch which has been broken down. 5. Why was amylase and starch kept separate while being allowed to reach the same temperature, before mixing? 6. On the basis of the data collected, describe and compare the rates of amylase catalysed reaction at different temperatures. (i.e. the effect of temperature on rate of reaction - use your graph to aid you). 7. Give two reasons why the rate of reaction increases with temperature up to a point. 8. How adequate is the colour change of iodine solution as an indicator of endpoint of the reaction? 9. How could you make the endpoint measurements more precise? Skills to be assessed: ORR and M&M Effect of Temperature on Enzymatic Rate of Reaction: SKILLS ASSESSED A: Observation, Recording and Reporting Table 1 Observation of colour of iodine as an indication of breakdown of starch by amylase at 5 different temperatures. Time (seconds) Temperature (°C) 60 100 25 Room temp. (28) 40 0 30 60…. 600 Table 2 Rate of hydrolysis of starch by amylase at different temperatures Mean time taken for complete Rate of reaction (min-1) Temperature (°C) hydrolysis of starch* (min) 25 28 (room temperature) 40 60 100 * Complete hydrolysis should be when the iodine does not produce any further colour change with starch/amylase mixture – it stays yellow brown, or if time does not permit, it stays red violet. Graph should have Title and Scale Labelled x axis (temperature °C) and y axis (rate of reaction min-1) [with units] Points with either a circled dot or an x Plotted points connected by straight lines B: Manipulation and Measurement Criteria 1. Working in groups - organisation Divide up and designate tasks within the group Read procedure and follow instructions successfully Performed at least 1 task with the group, not just standing and watching 2. Set up and maintain water bath at appropriate temperature, by manipulating heat from Bunsen burner. 3. Use of thermometer - measurement Interpreting accuracy of scale, Examines thermometer first and does not require teacher’s assistance to read thermometer Take reading while bulb is immersed, and at eye level 4. Use of thermometer – handling apparatus Immerse bulb completely in liquid Bulb does not make contact with container Stir liquid for even distribution of heat 5. Use of solutions Adding starch and amylase to test tubes to place in water bath before mixing Adding iodine to starch/amylase mixture after mixing starch and amylase Economical use of solutions – does not waste iodine Cleans tile properly for each temperature to be tested 6. Work area (start and end) Arrange apparatus (test tubes, test tube holders and Bunsen burner) for ease of use and in an uncluttered manner. Wash up all apparatus and wipe work area at the end of exercise. TOTAL Lower Six Biology – 2008 Marks 2 1 2 2 3 2 12