Arterial Input function Measurement for [11C]PHNO Arterial blood
advertisement
![Arterial Input function Measurement for [11C]PHNO Arterial blood](http://s3.studylib.net/store/data/007746397_2-54438de668d8c28a855ef4472b3c5526-768x994.png)
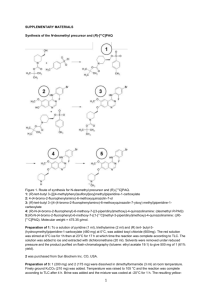
Arterial Input function Measurement for [11C]PHNO Arterial blood sampling was performed using a vascular access port (VAP), that is, a thin catheter leading from the femoral artery to a port implanted subcutaneously in the area of the hip, upper thigh or dorsum, during a previous surgery. Sequential discrete arterial blood samples were taken at 0.75, 1.5, 2.25, 3, 3.75, 4.5, 5.25, 6, 8, 10, 15, 20, 25, 30, 40, 50, 60, 75, 90, 105 and 120 min postinjection. Sample volumes ranged from 0.5 to 6 mL. Plasma was separated from blood cells by centrifugation (3900g for 5 min at 4ºC; Allegra X-22R Centrifuge, Beckman Coulter, Fullerton, CA, USA). Whole blood and plasma samples were counted in a cross-calibrated well counter (Wizard 1480, Perkin Elmer, Waltham, MA, USA). In order to measure the ligand metabolism in plasma, six selected plasma samples (3, 8, 15, 30, 60 and 90 min postinjection; 0.5 to 6 mL) were mixed with urea and citric acid at a final concentration of 8 M urea and 50 mM citric acid, and filtered through with a Millipore syringe filter (0.45 µm). The filtrate was then analyzed by reverse-phase HPLC using a column-switching system (Hilton et al, 2000) Up to 5 mL of filtrate was loaded on the HPLC system (Shimadzu, Kyoto, Japan), and a mobile phase of 1% acetonitrile water was eluted through the self-packed capture column with solid phase extraction C18 sorbent (Strata-X, Phenomenex, Torrance, CA, USA) at a flow rate 2 mL/min. Then the content of the capture column was back-flushed onto a Phenomenex Luna C18 analytical column (250x4.6mm, 5 µm) (Phenomenex, Torrance, CA, USA), with a mobile phase consisting of 77:23 0.1M ammonium formate: acetonitrile at a flow rate of 1.4 mL/min for the analytical column. The output of the HPLC column was connected to a fraction collector (CF-1 Fraction Collector, Spectrum Chromatography, Houston, TX, USA). Fractions were collected every two minutes and counted in a cross-calibrated well counter. The ratio of the radioactivity concentrations in filtrate and plasma was obtained and fitted to a exponential raise to plateau curve ( ). The unchanged fraction in the filtrate was fitted to an integrated gamma function: The unchanged fraction in plasma was then computed as the product of the functions and , and finally the arterial input function was computed as the production of the plasma radioactivity concentration and the unchanged fraction. [11C]PHNO was rapidly metabolized: the parent fraction ( ) was 32±3.5% at 30 min post injection and 10±2.3% at 90 min post injection (n=16). The fraction of radioactivity recovered after filtering the proteins was 94±1.6% at 3 min post injection and raised to 98±1.5% at 90 min post injection (n=16). Comparison of binding potentials BPND estimates for [11C]PHNO [11C]PHNO time-activity curves were fitted using the one- and two-tissue compartment (1TC and 2TC, respectively) models and the multilinear analysis MA1 (Ichise et al, 2002) using the arterial input function to estimate volume of distribution (VT) and binding potentials (BPND) (Innis et al, 2007) using the cerebellum as reference region to estimate the non-displaceable volume of distribution VND. In order to estimate binding potentials for studies for which arterial blood sampling was not available, binding potentials BPND were also estimated using SRTM (Lammertsma and Hume, 1996) and MRTMS using the cerebellum TAC as input data. MRTMS is based on MRTM and MRTM2 (Ichise et al, 2008; Ichise et al, 2003), except that it simplifies the equation by ignoring one term, in a manner identical to that of the simple form of the reference region graphical analysis (Gunn et al, 2002; Logan et al, 1996 Eq 7). The MRTMS operational equation is: where and represent the target ROI and cerebellum time-activity curves, respectively. The time after which the underlying graphical analysis plot is considered linear was set to 20 minutes for MA1 and 40 minutes for MRTMS. Representative fits of [11C]PHNO time-activity curves are shown in figure S1, with the corresponding 1TC and 2TC fits (figure S1A), MA1 fits (figure S1B), SRTM fits (figure S1C) and MRTMS fits (figure S1D). [11C]PHNO time-activity curves were not well fitted using the 1TC model (Figure S1A), and the two-tissue compartment model VT estimates were unreliable, with 35 out 96 VT estimates having percent standard errors (computed using the covariance matrix) exceeding 100%. MA1 provided acceptable fits (figure S1B) and the standard errors of MA1 VT estimates were lower than 13% in all regions and scans. Therefore, MA1 was selected as the gold standard method to estimate binding potentials BPND using arterial blood sampling and used to select the method to quantify binding potential using the reference region input. Though SRTM provided acceptable fits of [11C]PHNO time-activity curves, SRTM BPND values tended to underestimate [11C]PHNO BPND from MA1 (figure S2A), especially in high binding regions. The slope of the regression line of the correlation plot between MA1 and MRTMS BPND estimates (figure S2B) was closer to unity than for the correlation plot between MA1 and SRTM BPND estimates (slope=0.893 for MRTMS, slope=0.786 for SRTM). Similarly, the intercept of regression line of the correlation plot between MA1 and MRTMS BPND estimates was lower (intercept=0.244 for MRTMS, intercept=0.549 for SRTM). Therefore, MRTMS was selected to quantify [11C]PHNO binding potential using cerebellum time-activity curves as input data. Figure S1: Representative time-activity curves from one baseline [11C]PHNO scan in the caudate nucleus (solid circles), putamen (open circles), pallidum (open diamonds), substantia nigra (open triangles), nucleus accumbens (open squares), and their fitted model curves) using 1TC (dotted lines) and 2TC (solid lines) models (A), MA1 (B), SRTM C) and MRTMS (D). B 10 10 8 8 MRTMS BPND [ unitless ] SRTM BPND [ unitless ] A 6 4 2 6 4 2 0 0 0 2 4 6 8 10 MA1 BPND [ unitless ] 0 2 4 6 8 10 MA1 BPND [ unitless ] Figure S2: Correlation between BPND estimated using reference-input based methods and MA1. Dotted lines represent the line of identity. (A) Correlation between SRTM and MA1 BPND estimates (16 scans, 5 regions). The equation of the regression line (solid line) is y=0.786x+0.549, r2=0.961. (B) Correlation between MRTMS and MA1 BPND estimates (16 scans, 5 regions). The equation of the regression line (dashed line) is y=0.893x+0.244, r2=0.962. MRTMS BPND estimates are in better agreement with MA1 BPND estimates than SRTM BPND estimates. References Gunn RN, Gunn SR, Turkheimer FE, Aston JAD, Cunningham VJ (2002). Positron emission tomography compartmental models: a basis pursuit strategy for kinetic modeling. 22(12): 1425-1439. Hilton J, Yokoi F, Dannals RF, Ravert HT, Szabo Z, Wong DF (2000). Column-switching HPLC for the analysis of plasma in PET imaging studies. 27(6): 627-630. Ichise M, Cohen RM, Carson RE (2008). Noninvasive estimation of normalized distribution volume: application to the muscarinic-2 ligand [(18)F]FP-TZTP. 28(2): 420-430. Ichise M, Liow J-S, Lu J-Q, Takano A, Model K, Toyama H, et al (2003). Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. 23(9): 1096-1112. Ichise M, Toyama H, Innis RB, Carson RE (2002). Strategies to improve neuroreceptor parameter estimation by linear regression analysis. 22(10): 1271-1281. Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al (2007). Consensus nomenclature for in vivo imaging of reversibly binding radioligands. 27(9): 1533-1539. Lammertsma AA, Hume SP (1996). Simplified reference tissue model for PET receptor studies. 4(3 Pt 1): 153-158. Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL (1996). Distribution volume ratios without blood sampling from graphical analysis of PET data. 16(5): 834-840.






![ACCURACY OF [11C] CHOLINE POSITRON EMISSION](http://s3.studylib.net/store/data/006910188_1-178035aba028502f62a71ecfd059e7d4-300x300.png)
