display
advertisement
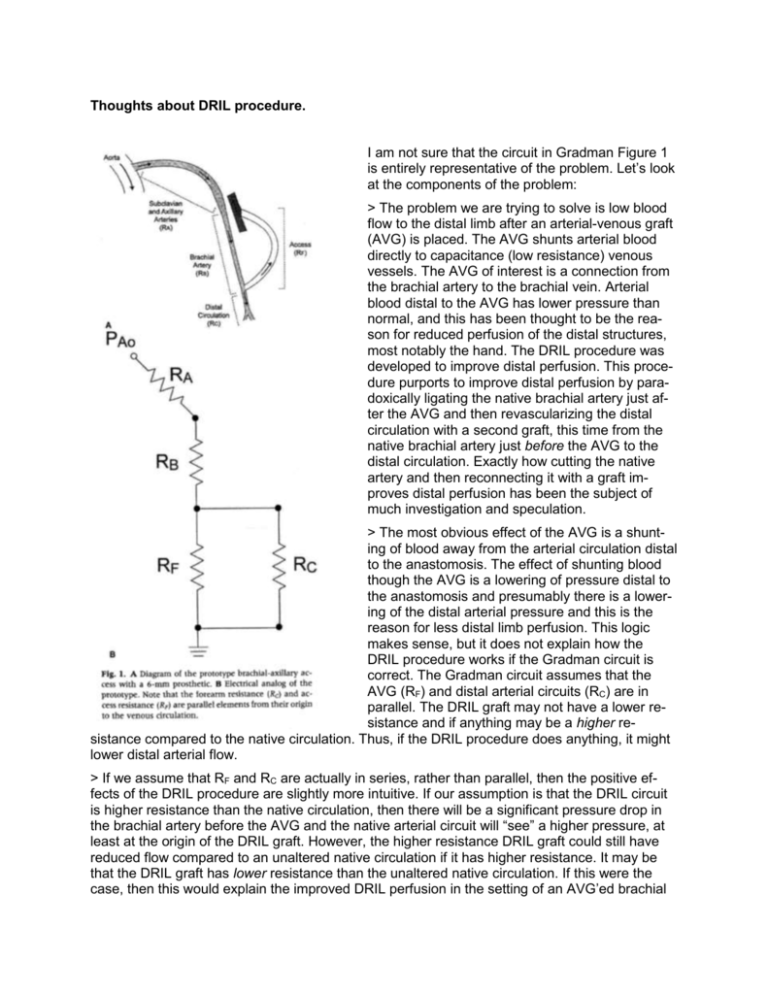
Thoughts about DRIL procedure. I am not sure that the circuit in Gradman Figure 1 is entirely representative of the problem. Let’s look at the components of the problem: > The problem we are trying to solve is low blood flow to the distal limb after an arterial-venous graft (AVG) is placed. The AVG shunts arterial blood directly to capacitance (low resistance) venous vessels. The AVG of interest is a connection from the brachial artery to the brachial vein. Arterial blood distal to the AVG has lower pressure than normal, and this has been thought to be the reason for reduced perfusion of the distal structures, most notably the hand. The DRIL procedure was developed to improve distal perfusion. This procedure purports to improve distal perfusion by paradoxically ligating the native brachial artery just after the AVG and then revascularizing the distal circulation with a second graft, this time from the native brachial artery just before the AVG to the distal circulation. Exactly how cutting the native artery and then reconnecting it with a graft improves distal perfusion has been the subject of much investigation and speculation. > The most obvious effect of the AVG is a shunting of blood away from the arterial circulation distal to the anastomosis. The effect of shunting blood though the AVG is a lowering of pressure distal to the anastomosis and presumably there is a lowering of the distal arterial pressure and this is the reason for less distal limb perfusion. This logic makes sense, but it does not explain how the DRIL procedure works if the Gradman circuit is correct. The Gradman circuit assumes that the AVG (RF) and distal arterial circuits (RC) are in parallel. The DRIL graft may not have a lower resistance and if anything may be a higher resistance compared to the native circulation. Thus, if the DRIL procedure does anything, it might lower distal arterial flow. > If we assume that RF and RC are actually in series, rather than parallel, then the positive effects of the DRIL procedure are slightly more intuitive. If our assumption is that the DRIL circuit is higher resistance than the native circulation, then there will be a significant pressure drop in the brachial artery before the AVG and the native arterial circuit will “see” a higher pressure, at least at the origin of the DRIL graft. However, the higher resistance DRIL graft could still have reduced flow compared to an unaltered native circulation if it has higher resistance. It may be that the DRIL graft has lower resistance than the unaltered native circulation. If this were the case, then this would explain the improved DRIL perfusion in the setting of an AVG’ed brachial artery. The DRIL graft has greater diameter than a native brachial artery, and this could explain lower resistance, but one would think that the effects of the anastomoses might negate any reduction in resistance by the graft diameter. Certainly, the effects of DRIL graft resistance (diameter and length) are areas that should be investigated thoroughly. > The purpose of the AVG is to produce a high-flow shunt that can be tapped repeatedly for the purposes of dialysis. The AVG is an arterial to venous graft for two reasons: (1) A-V grafts have a high pressure difference and thus always have high flow. The high flow is required so that there is an ample supply of blood for the dialysis machine and so that thrombus does not form in the graft when not in use. (2) The discharge from the dialysis machine enters the venous circulation directly, without any intervening capillary bed. If the dialysis machine was connected to an arterial circuit or an AA graft, then the output of this machine would directly encounter a capillary bed. The advantage of direct venous drainage is filtration of any impurities by the pulmonary vascular bed. Without this, embolic ischemic events would likely not be uncommon in dialysis patients. A secondary effect of the AVG high flow and low resistance is a local increase in the venous pressure. I suspect that this increase in venous pressure is a major component of the reduced peripheral perfusion seen after AVG placement. Normal central venous pressure is in the range of 5 mmHg. Venous pressure increases as the vertical distance between the measurement point and the heart increases. In the upright position, the hand is roughly 15 in or 38 cm lower than the heart. This corresponds to an increase in peripheral venous pressure to 28 mmHg over central venous pressure, or a local venous pressure in the range of 33 mmHg in the hand under normal circumstances. Add a nearly direct arterial-venous connection to this already high venous pressure, and local venous pressure in the hand probably doubles or more than doubles. Most dialysis patients are hypertensive, so this would increase the venous pressure even further. Figure 1 in the Gradman paper assumes that the venous pressure is the same for the AVG and the peripheral arterial circuit (both RF and RC are connected to a common ground) and I think that this is a mistake. The venous pressure for the AVG is lower than the peripheral arterial circuit due to the height differences relative to the heart and due to the effects of the AVG itself. The effect of venous pressure on peripheral arterial perfusion needs to be better investigated (it would be great to have some in vivo data here). > The DRIL procedure effectively adds a length of brachial artery to the AVG. This increases the effective resistance of the AVG based on the diameter and length of brachial artery beyond the DRIL anastomosis. In effect, the length brachial artery before the AVG anastomosis is like banding the fistula. None of the 3 articles given to us measure flow in the AVG before and after the DRIL procedure. These articles studied symptomatic AVG patients and documented improved pressure and perfusion in the distal arterial circuit, but they did not measure flow in the AVG or measure the resistance of the AVG after DRIL. This is an area that should be explored. > I think that most clinicians would think that the peripheral vascular resistance in the circulation distal to the AVG would be either unchanged or lower than what it was before the AVG. If the perfusion pressure of the distal circulation is lower after the AVG, then common sense would say that there should be a corresponding decrease in peripheral vascular resistance to compensate and to try to maintain local flow. However, it might be opposite; the peripheral vascular resistance in the hand may be higher after the AVG placement. This could occur because of a reduction of NO and other vasoactive substances cased by the high flow and dilation of the vessels proximal to the AVG. These vasoactive substances could increase the peripheral vascular resistance of both the arterial circulation to the hand and also increase the venous vascular resistance at the exit of the AVG. Both of these effects would decrease the perfusion of the hand and could be progressive (ie: these effects might become more progressive over time). This is an area that should be explored with a clinical study. Thoughts about an experimental procedure. > The electrical circuit diagram of Gradman could be modified to better characterize the hemodynamics of the DRIL circuit (see below): The proposed new circuit diagram is a variation on Gradman Figure 1. In this new proposal, the resistance of the brachial artery segment proximal to the AVG is added as is the resistance of the DRIL and a venous resistance is added. The venous resistance accounts for the additional venous pressure that is the result of the excess flow induced by the AVG and by the excess pressure added to the venous system by the direct arterial-venous shunt. > The tubing connections need to be without obstruction. This eliminates most connectors with the exception of thin stainless steal tubing, which has extremely thin wall thickness. Alternatively, the connections can be sewn, but this would require some surgical training and would be associated with some leakage. > The tubing should be surgical graft material of appropriate sizes (an experimental variable). > The experimental flow system should be in parallel with the main flow in the system (see below). The critical feature of this model is that not all the output of the heart pump needs to traverse the experimental circuit. My suspicion is that requiring all flow to traverse the experimental circuit will overwhelm the circuit and force flow patterns that might not otherwise exist. This will mean that we will have to have an appropriate venous pressure in the venous reservoir. > We will set the systemic vascular resistance and heart pump rate/stroke volume. This will set a baseline cardiac output and arterial pressure. All of the variables will be in the experimental circuit. > A tapering graft will be used for the brachial artery. One of the variables will be the length of brachial artery included in the AVG segment. > The diameter and length of the AVG and DRIL will be variables. > The venous outlet of the experimental circuit should also be a graft of fixed length and diameter. > Three experimental setups are proposed. 1. The Native Circulation will be used to measure baseline pressures and flows and for the calculation of segmental and total resistance. The peripheral circulation (a sponge slice in a flow chamber) will be varied to achieve clinically relevant values and then not changed for the remainder of the experimental plan. NOTE: We might want to determine the sponge size that doubles baseline flow, as the normal hemodynamic response to AVG is to lower peripheral vascular resistance through vaso-dilation and vaso-recruitment. 2. The AVG, no DRIL will be used to assess the pressures and flows of the standard AV graft. Different lengths of AVG and different attachment points to the brachial artery will be tried. The goal is determine what effect changes in AVG size (diameter and length = resistance) have on the perfusion pressure and flow in the peripheral circulation. The arrangement with the worse peripheral flow characteristics will be used for the actual DRIL experiments. 3. The AVG w/ DRIL will be used for the actual testing of the DRIL theory. Here the variables are DRIL size (diameter, not length - this change adds and subtracts resistance to the DRIL circuit), proximal attachment point (axillary artery or brachial artery just above the AVG - this change adds and subtracts resistance to the AVG circuit). Low peripheral flow is usually not noted until late after AVG placement (months, not days). This suggests that dilation of the AVG over time results in reduced graft resistance and a corresponding increase in venous pressure. > A thorough assessment of the AVG, no DRIL and AVG, w/ DRIL circuits should be done so that the experimental data can be compared to calculated data.