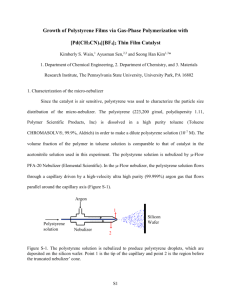
replace this with the actual title using all caps
advertisement

CHEMICAL MODIFICATION OF POLYSTYRENE AND GOLD SURFACES A Thesis Presented to the Faculty of the Graduate School of Cornell University In Partial Fulfillment of the Requirements for the Degree of Master of Engineering by Alexander David Roth August 2009 © 2009 Alexander David Roth ABSTRACT Investigations toward the surface modification of gold and polymers and the long-term storage of functionalized surfaces were carried out. First gold was modified with cystamine to see if amine functional groups could be formed on gold surfaces. Data were inconclusive as an acid orange 7 assay determined low amine group coverage but Schiff’s reagent (fuscin-sulfurous acid) test showed a subsequently higher aldehyde group coverage when coupling glutaraldehyde to the aminated surface. Second, biotin and Ru(bpy3)2+ were conjugated using NHS-ester and amine conjugation chemistry, however, no coupling was observed. Third, carboxylic acid groups were generated on polystyrene (PS) and polymethyl methacrylate (PMMA) using UV and ozone treatment and quantified using water contact angle to measure hydrophobicity, and utilizing a toluidine blue O assay for the carboxylic acid surface concentration. Longterm storage under various storage conditions including temperature, buffer components and pH were tested. An initial water contact angle of 42.0o and carboxylic acid group density of 12.4 nmol/cm2 were obtained for PS. In general, even under best conditions, an initial loss of functionality was observed reaching a steady state condition after 1 hour of storage. It was also determined that the temperature of storage did not affect hydrophobicity or carboxyl surface concentration. Samples stored in dry conditions maintained a significantly higher surface concentration than samples stored in 0.1 M phosphate buffer. PMMA retained 100 % surface functionality after heating up to 90oC, 95oC, and 100oC, while becoming more hydrophobic, indicating the loss of functional groups that are not carboxylic acid. Conversely, heated polystyrene did not become hydrophobic, but lost about 40% of its carboxyl surface groups. Samples coated with acetyl acetone were very hydrophobic, and carboxyl surface concentration was inconsistent. Hydrophobicity tended to increase with pH for HEPES, Tris, and sodium borate buffer, with water and Tris maintaining fairly hydrophilic surfaces (52.5-55.9o). However, the best carboxyl surface concentration was achieved on pH 5 and 7 water and pH 5 PBS. Thus, the best conditions for storage are dry stored conditions, acidic and neutral water, or acidic PBS buffer. ii BIOGRAPHICAL SKETCH Alexander David Roth graduated from Oceanside High School in June of 2005. Following this, he went to pursue a B.S. in Biological and Environmental Engineering at Cornell University. Alex completed his B.S. by January 2009, and went on to do his Master’s of Engineering in Agricultural and Biological Engineering at Cornell University. While at Cornell, Alex was treasurer of the Cornell Chapter of Institute of Biological Engineers and participated as a peer advisor for the College of Engineering. Additionally, Alex served as a teaching assistant for bioengineering kinetics and thermodynamics course for two semesters. In the summer of 2007, Alex performed research at Cornell Weill Medical College in New York City under the direction of Dr. Baolin Wang of the Department of Genetic Medicine. There, Alex performed research on analyzing the sonic hedgehog (Shh) pathway, and how mutations in the pathway can cause neurological and skeletal mutations in mice and people. In the summer of 2008, Alex performed research at Roswell Park Cancer Institute under the direction of Dr. Daryl Nazareth in the department of Medical Physics. Alex’s research involved optimization of radiation treatment applied to prostate cancer patients using intensity modulated radiation therapy. Alex is currently performing research under the direction of Dr. Antje J. Baeumner in the Department of Biological and Environmental Engineering at Cornell University. In his research, Alex analyzed surface modification of gold and polystyrene, and how these modifications can be effected based on thermal and chemical treatments, and how storage conditions may also affect surface treatment. 3 To my parents, Jeffrey and Ronnie Roth, my first real teachers. 4 ACKNOWLEDGMENTS This work was done with the help of the National Institute of Health (NIH) and the Cornell Nanobiotechnology Center (NBTC). Major contributors to the success of this research include Antje J. Baeumner, Sam R. Nugen, and Peter J. Asiello. 5 TABLE OF CONTENTS Chapter 1: Functionalization of Gold Plated Surfaces 12 1.1 Introduction 12 1.2 Materials and Methods 13 1.2.1 Amination of Gold Surface 13 1.2.2 Acid Orange 7 (AO7) Assay for Quantification of 13 Amine Surface Concentration 1.2.3 Glutaraldehyde Conjugation on Aminated Gold 14 Surfaces 1.3 Results and Discussion 15 1.4 Conclusions and Future Work 16 Chapter 2: Electrochemiluminescence 20 2.1 Introduction 20 2.2 Materials and Methods 22 2.2.1 Conjugation of Biotin and Ruthenium 22 2.2.2 ECL Readings Using Streptavidin-Coated Beads 22 2.3 Results and Discussion 23 2.4 Conclusions and Future Work 25 Chapter 3: Long-Term Stability of Hydrophobicity and Carboxyl 29 Functionality on Polystyrene Surfaces 3.1 Introduction 29 3.1.1 Polystyrene: Design of a Microfluidic Device 29 3.1.2 Stability of UV and Ozone Treated Surfaces 29 6 31 3.2 Materials and Methods 3.2.1 Sample Preparation 31 3.2.2 Water Contact Angle Analysis 31 3.2.3 Toluidine Blue O (TBO) Assay for Quantifying 32 Carboxyl Surface Concentration 3.3 Results and Discussion 33 3.3.1 Analysis of Controls 33 3.3.2 Forty Eight Hours after Initial Treatment 35 3.3.3 Seven Days after Initial Treatment 37 3.3.4 Four Weeks after Initial Treatment 39 3.4 Conclusions and Future Work 41 Chapter 4: The Affects of Heating, Surface Coating, and Buffer Storage 47 on Hydrophobicity and Carboxyl Functionality of UV-Treated Polystyrene and PMMA Surfaces 4.1 Introduction 47 4.1.1 PMMA: Design of a Microfluidic Device 47 4.1.2 Thermal and Chemical Effects on Carboxyl Surface 48 Chemistry and Hydrophobicity 4.2 Materials and Methods 50 4.2.1 Sample Preparation 50 4.2.2 Water Contact Angle Analysis 51 4.2.3 Toluidine Blue O (TBO) Assay for Quantifying 51 Carboxyl Surface Concentration 4.3 Results and Discussion 51 4.3.1 PMMA and Polystyrene: Thermal Bonding 55 4.3.2 Polystyrene and Solvent Bonding 55 7 4.3.3 Buffer and pH Experiments 4.4 Conclusions and Future Work 56 58 8 LIST OF FIGURES Figure 1.1: AO7 Calibration for quantifying amine surface concentration 15 Figure 1.2: Quantification of amine surface concentration on gold plates 16 Figure 2.1: Desired conjugation of NHS-PEG4-Biotin with 23 Ru(bpy)2(phen-5-NH2)(PF6)2 Figure 2.2: Conjugation product of biotin and ruthenium in the presence of 24 DMSO Figure 2.3: ECL detection levels of pure streptavidin, biotin and 25 ruthenium, and streptavidin bound to biotin and biotin-ruthenium conjugate product Figure 3.1: Calibration curve for determination of carboxyl surface 34 concentration on UV treated polystyrene surfaces Figure 3.2: Average water contact angles for non-stored polystyrene 35 Figure 3.3: Average carboxylic acid surface concentration for non-stored 36 polystyrene Figure 3.4: Average water contact angle for polystyrene 1 hr, 5 hr, 24 hr, 36 and 48 hr after UV and ozone treatment Figure 3.5: Average carboxyl surface concentration for polystyrene 1 hr, 5 37 hr, 24 hr, and 48 hr after UV and ozone treatment Figure 3.6: Average water contact angle for polystyrene 24 hr, 48 hr, 4 38 days, and 7 days after UV and ozone treatment Figure 3.7: Average carboxyl surface concentration for polystyrene 24 hr, 39 48 hr, 4 days, and 7 days after UV and ozone treatment Figure 3.8: Average water contact angle for polystyrene 7 days, 14 days, 3 weeks, and 4 weeks after UV and ozone treatment 9 40 Figure 3.9: Average carboxyl surface concentration for polystyrene 7 40 days, 14 days, 3 weeks, and 4 weeks after UV and ozone treatment Figure 4.1: Water contact angle analysis for PMMA control samples 52 Figure 4.2: Water contact angle analysis for UV and Ozone treated 53 PMMA and polystyrene heated samples Figure 4.3: Carboxylic acid surface quantification for PMMA control 54 samples Figure 4.4: Carboxylic acid surface quantification for UV and Ozone 55 treated PMMA and polystyrene heated samples Figure 4.5: Water contact angle analysis for polystyrene with a surface 56 coating of acetyl acetone Figure 4.6: Carboxylic acid surface quantification for polystyrene with a 57 surface coating of acetyl acetone Figure 4.7: Water contact angle analysis for UV and ozone treated 58 polystyrene stored in various solutions for 1 hour at either acidic, neutral or basic pH Figure 4.8: Carboxylic acid surface quantification for UV and ozone treated polystyrene stored in various solutions for 1 hour at either acidic, neutral or basic pH 10 59 LIST OF TABLES Table 3.1: Average water contact angles for UV and ozone treated 43 polystyrene samples stored over a four week duration Table 3.2: Average carboxyl surface concentration for UV and ozone 44 treated polystyrene samples stored over a four week duration Table 4.1: Average water contact angles on the surface of heated and UV 61 and ozone treated polystyrene and PMMA Table 4.2: Average carboxylic acid surface concentration on the surface of 61 heated and UV and ozone treated polystyrene and PMMA Table 4.3: Average Water Contact Angles for UV and Ozone Treated 62 Polystyrene samples stored in various buffers at acidic, neutral, or basic pH Table 4.4: Average carboxylic acid surface concentration for UV and Ozone Treated Polystyrene samples stored in various buffers at acidic, neutral, or basic pH 11 62 CHAPTER 1 Functionalization of Gold-Plated Surface 1.1: Introduction Thiols are often used for functionalizing gold surfaces, facilitating the immobilization of biomolecules onto gold1. However, amine modification is interesting because it can utilize the disulfide bond for gold attachment while expanding the kinds of molecules that can be immobilized onto gold surfaces. Cystamine (C4H12N2S2) and cysteamine (C2H7NS) are compounds that utilize a disulfide bond for attachment of amine groups2. This is important as amines are often used as a base for the attachment of other molecules3. Cystamine has been used in the past to immobilize many oxidationreduction sensitive proteins2. N-Hydrosuccinimide (NHS) esters are often conjugated to amine groups as a way to covalently couple proteins onto surfaces4. Additionally, amines can be easily conjugated with most protein functional groups and peptides groups, especially those with carboxylic acid functional groups4. In addition to amine conjugation with carboxylic acids being useful toward immobilization of proteins, amines can conjugate with other amines via linker molecules. Aldehyde and amine conjugation chemistry is well studied, and glutaraldehyde makes a good linker between amine functional groups5 and also in the development of polymers6. Additionally, the use of glutaraldehyde as a linker has allowed for use of immobilization of amino acids, peptides, and proteins7,8. This is important for the surface immobilization of detection proteins such as streptavidin (which will be discussed more in the following section and in chapter 2). 12 In the experiments described, amine was functionalized onto gold surfaces using cystamine as the conjugation solvent. Following cystamine conjugation, aldehyde groups were conjugated to the amine surfaces and qualitatively analyzed to determine if aldehyde conjugation was successful. An acid orange 7 (AO7) assay was used to quantify the amine functionalization on the gold surface and compared to amine quantification using similar protocols. 1.2: Materials and Methods 1.2.1: Amination of Gold Surface Gold surfaces were functionalized according to a protocol used by Wirde and Gelius2. Before functionalization, all gold slides were cleaned by soaking 1 hr in 98% ethanol. Samples were washed with deionized water and dried with N2 following the ethanol soak. This was followed by exposing the gold surfaces to 254 nm UV light using a Jelight UVO-Cleaner, model# 144AX. The machine had a power of 28 mW/cm2 while the light source emitted light at approximately 1cm away from the surface. Samples were exposed for fifteen minutes followed by a two minute flush with air. Gold slides were soaked in a 2.5 mM cystamine in water solution for twenty-four hours. Following functionalization, cystamine slides were rinsed with ethanol and dried with N2. 1.2.2: Acid Orange 7 (AO7) Assay for Quantification of Amine Surface Concentration 13 Gold slides were immersed in a 1.0 mM AO7 solution in pH 12 water. The gold slides remained immersed in the solution for three hours at room temperature. After the immersion, the gold slides were rinsed three times with pH 12 water and left to dry. After the slides were dried, the acid orange 7 dye was desorbed by placing the gold surfaces on pH 3 water and shaking for 15 minutes. Absorbance was measured using a spectrophotometer. A standard curve was created for 100 µM, 10 µM, 5 µM, 1 µM, 500 nM, and 100 nM concentrations of AO7. Absorbance was measured at a wavelength of 460 nm. Surface concentration was calculated using the following equation: (1) A = p*c Where A is the absorbance, c is the concentration, and p is a constant. A sample calibration of an AO7 assay can be seen in figure 1.1 1.2.3: Glutaraldehyde Conjugation on Aminated Gold Surfaces The amine-gold surface was subsequently coupled to glutaraldehyde. A protocol for conjugation was used similar to that in the bioconjugate techniques book12. Gold slides were immersed in 0.1 M pH 9.5 sodium borate (NaBO3) buffer. Glutaraldehyde was added to make a 1:1 molar ratio of aldehyde to amine functional groups. Sodium cyanoborohydride (NaCNBH3) was added to form a 0.05M solution of NaCNBH3 in sodium borate buffer. The reaction was allowed to proceed for two hours at room temperature. Slides were removed from solution and rinsed with deionized water and dried with N2. 1 mL of Schiff’s reagent was applied to each surface to determine the 14 Acid Orange 7 Calibration y = 15327x R2 = 0.9998 Absorbance at 460 nm 10 1 0.1 0.01 0.001 0.0000001 0.000001 0.00001 0.0001 AO7 Concentration (M) Figure 1.1 AO7 Calibration for quantifying amine surface concentration. Concentration of amines is equal to concentration of dye. Surface concentration can be calculated by multiplying the dye concentration by the desorbing volume and dividing by the area of desorption. strength of aldehyde functionalization on the gold surface. The color change observed determined the concentration of aldehydes present on the gold surface. 1.3: Results and Discussion Ultimately, amination of the gold surface was successful using cystamine. However, the maximum surface concentration was not attained with these experiments (see figure 1.2). While amine surface chemistry did increase when gold was in the presence of cystamine, the value 0.145 nmol/cm2 is less than the 1 nmol/cm2 obtained by other groups2. This conjugation concentration was even low compared to the total concentration of amines on gold after 1 hour of a cystamine in gold soak2. Nonetheless, the low surface concentration may not be a problem. If large protein molecules bond to the surface amine groups on gold, intermolecular forces may allow proteins to spread out over the electrode surface. 15 Surface Concentration (nmol/cm^2) Amine Surface Chemistry on Gold 0.18 0.16 0.14 0.12 0.1 0.08 0.06 0.04 0.02 0 No Cystamine Cystamine Treatment Figure 1.2 Quantification of amine surface concentration on gold plates. Schiff’s Reagent should turn magenta or purple in the presence of a high concentration of aldehydes10. The application of Schiff’s reagent to surfaces plated with cystamine and glutaraldehyde caused a dark blue color to be immediately produced. This indicates a very strong presence of aldehydes (though the quantification of the amount of aldehydes is unknown). This may mean that that amount of desorbed acid orange 7 could have underestimated the amine surface concentration on the gold slides. 1.4: Conclusions and Future Work Amination of the gold surface was successful using 2.5 mM cystamine in water. However, the results of the AO7 assay also indicate that the maximum surface concentration of amines was not reached2. The results of the Schiff’s reagent test indicate a strong presence of aldehydes on the gold surface. The result of the very successful conjugation between the aminated gold surface and glutaraldehyde 16 indicates that the acid orange 7 assay may have underestimated the total surface concentration of amine functional groups. A future test might be to get a different read on the amine surface chemistry on gold using a different test. It is also possible that if the amine concentration calculated by the AO7 assay is accurate, the amine surface concentration may be sufficient to immobilize proteins or peptides onto the gold surface. Intermolecular forces and steric effects will limit the amount of amines that can conjugate with proteins. Using glutaraldehyde as a linker molecule will also have a minor impact on the total concentration of protein that can bind to the gold surface. 17 REFERENCES 1. Hostetler, M.J., Stokes, J.J., Murray, R.W. Infrared Spectroscopy of ThreeDimensional Self-Assembled Monolayers: N-Alkanethiolate Monolayers on Gold Cluster Compounds. Langmuir, 12, 1996. pp. 3604-3612. 2. Wirde, M., Gelius, U. Self Assembled Monolayers of Cystamine and Cysteamine on Gold Studied by XPS and Voltammetry. Langmuir, 15, 1999. pp. 6370-6378. 3. Doron, A., Katz, E., Willner, I. Organization of Au Colloids as Monolayer Films onto ITO Glass Surfaces: Applications to the Metal Colloid Films as Base Interfaces to Construct Redox-Active Monolayers. Langmuir, 11, 1995. pp. 1313-1317. 4. Abello, N., Kerstjen, H.A.M., Postma, D. Selective Acylation of Primary Amines in Peptides and Proteins. Journal of Proteome Research, 6, 2007. pp. 4770-4776. 5. Chang, M.C., Tanaka, J. FT-IR Study for Hydroxyapatite/Collagen Nanocomposite Cross-linked by Glutaraldehyde. Biomaterials, 23, 2002. pp. 4811-4818. 6. Hardy, P.M., Nicholls, A.C., Rydon, H.N. Nature of Glutaraldehyde in Aqueous Solution. Chemical Communications, 1969. 565-566. 7. Hopwood, D., Allen, C.R., McCabe, M. The Reactions Between Glutaraldehyde and Various Proteins; An Investigation of Their Kinetics. Histochemical Journal, 2, 1970. pp. 137-150. 8. Hopwood, D. Theoretical and Practical Aspects of Glutaraldehyde Fixation. Histochemical Journal, 4, 1972. pp. 267-303. 9. Hermanson, G.T. Bioconjugate 10. Techniques; 2nd Edition. 2008. pp. 265-268. 18 11. Bourne, G.H., Danielli, J.F. International Review of Cytology: The Chemistry of Schiff’s Reagent. 10, 1961. pp. 1-93. 19 CHAPTER 2 Electrochemiluminescence 2.1: Introduction ECL is the application of voltage in the presence of a luminescent molecule, which causes the emission of light1. During an ECL reaction, ruthenium complex usually in the form of tris(bipyridine) ruthenium (II) (Ru(bpy3)2+) emits a photon of light at a wavelength of 620 nm. This emission is caused by the excitation after ruthenium is reduced by tripropylamine (TPA) during the application of an electric potential. TPA and ruthenium are oxidized on the electrode. TPA then loses a proton, forming TPA radical, the form of TPA that ultimately reduces ruthenium to its excited state. Since the state of ruthenium is heavily tied to the presence of an applied potential, ECL has become popular for detection and modeling systems. Ruthenium does not require a light source or radiation for excitation, and it can be used for fairly low levels and a wide range of detection2. Additionally, ruthenium has been used in the detection of amine functional groups, with stronger reactions occurring in the presence of tertiary amines3. However, a stronger detection can be obtained by functionalizing the ruthenium protecting group with other functional groups. This is necessary as many biological molecules lack tertiary amine groups for necessary detection4. Often, Ru(bpy3)2+ will be produced with a NHS ester functionalized onto the protecting group of ruthenium5, as NHS-ester and amine bonding is well studied6. The NHS-ester linkage allows for ruthenium to be conjugated directly onto amino acids, 20 allowing it to be used as a protein label7. Ruthenium may also be produced with other functional groups to conjugate with other molecules that are useful for detection studies. One molecule that ruthenium has been conjugated to is PEG-biotin. Biotin has a very strong binding affinity for streptavidin, with a KD or dissociation constant of around 10-15 M8. In addition, biotin may have high affinity for other proteins (such as avidin), though streptavidin is most often used for binding with biotin. The process of modifying a molecule by conjugation with biotin (known as biotinylation) has been used for peptide and protein modification and detection9. Streptavidin and biotin binding chemistry can even hold well in the presence of strong detergents, which could otherwise damage cellular proteins or cause the lysis of a cell or liposome10. This strong binding chemistry between biotin and streptavidin under cellular lytic conditions can mean efficient purification of biotinylated molecules11. While this may pose a problem for further protein purification, it is very convenient for detection purposes, which rely on the strong binding affinity for measurement of sensitivity. In this experiment, ruthenium was conjugated to biotin utilizing standard NHS-ester and amine conjugation reactions. Four biotin molecules can bind to one streptavidin molecule12. The coupling of streptavidin with a surface protein and biotin with ruthenium allows for long term detection of ECL. Following a biotin-ruthenium conjugation, ECL measurements were taken, comparing the level of ECL between streptavidin, the biotin-ruthenium conjugation, and the biotin-ruthenium conjugate bound to the streptavidin. 21 2.2: Materials and Methods 2.2.1: Conjugation of Biotin and Ruthenium NHS-PEG4-biotin was conjugated to bis(2,2’-bipyridine)-(5aminophenanthroline)ruthenium bis (hexafluorophosphate) (Ru(bpy)2(phen-5NH2)(PF6)2), using NHS-ester and amine binding chemistry6. The ruthenium was diluted to 11.1 mM in PBS pH 7.2. Biotin was added to a concentration of 6.8 mM. The mixture was lightly shaken for two hours at room temperature. The conjugate was stored at -20oC for further studies. A second conjugation test was used, dissolving dimethyl sulfoxide (DMSO) to a concentration of 2.1mM in the PBS before adding the ruthenium. This was done to assist in the dissolving of the ruthenium complex in solution (see figure 2.1). 2.2.2: ECL Readings using Streptavidin-Coated Beads To determine the effectiveness of the conjugation, ECL readings were taken of the conjugates in the presence of Dynal Myone T1 streptavidin beads. Beads were separated out of solution with magnets for one to two minutes, with the solution being removed after separation. Beads were resuspended in PBS pH 7.2 to the same volume. The beads were separated out, and the PBS buffer was removed. This was repeated for a total of three PBS washes. Following the last wash, the biotin-ruthenium conjugate in solution was added to the beads. Conjugates were diluted to a concentration of 400 nM. The conjugates and the beads were gently shaken for 15 minutes at room temperature. After shaking, the beads and attached conjugates were separated from the supernatant using magnets. The supernatant was discarded, and the beads were resuspended in B+W buffer, which contained 5 mM Tris and HCl, 0.5 mM 22 ethylenediaminetetraacetic acid (EDTA), and 1 M NaCl at pH 7.5. This was repeated for a total of five washes. Samples were diluted 1/60 in a solution containing PBS, TPA, and TritonX-100 for ECL readings. ECL readings were measured using an Organon Teknika Nuclisens Reader. Samples were flushed with cleaning buffer containing KOH and TritonX between readings. Samples analyzed include a “blank” sample, streptavidin beads independent of biotin and ruthenium, conjugates by themselves, and bead-conjugate couples. All ECL readings were outputted to a computer. The strength of the reading is directly linear to the total concentration of ruthenium present in the solution. 2.3: Results and Discussion During the second conjugation experiment, the ruthenium was removed from its protecting group by application of the DMSO (see figure 2.2). Thus, while the + NHS-PEG4-biotin Ru(bpy)2(phen-5-NH2)(PF6)2 PBS Buffer, pH 7.2 2.5 hour shake at room temp Biotin-Ruthenium Conjugate Figure 2.1 Desired conjugation of NHS-PEG4-Biotin with Ru(bpy)2(phen-5-NH2)(PF6)2. 23 conjugation might have been successful, ECL would not have been detected Results of the ECL test show that ruthenium was not fully conjugated to biotin (see figure 2.3). If conjugation was successful, ECL readings for conjugated samples with streptavidin should have been much greater than that of pure ruthenium conjugate. However, the results show that some of the conjugation was successful because the average ECL value of the ruthenium conjugate in the presence of biotin was still about five times greater than that of pure streptavidin. Figure 2.2 Conjugation product of biotin and ruthenium in the presence of DMSO. The clear orange layer represents the PBS and DMSO solution where the reaction was performed. The opaque orange layer is the biotin conjugated to the protecting group of ruthenium. At the bottom is a small black layer, which is ruthenium without the protecting group 24 ECL Readings 2500 ECL Value 2000 1500 1000 500 0 Streptavidin Conjugate Streptavidin + Conjugate Sample Contents Figure 2.3: ECL detection levels of pure streptavidin, biotin and ruthenium, and streptavidin bound to biotin and biotin-ruthenium conjugate product. 2.4: Conclusions and Future Work Since NHS-ester and amine surface chemistry is well studied, the likely event is that the conjugation did not occur as a result of other factors affecting the experiment. While DMSO did dissolve ruthenium more quickly into PBS, it coincided with the separation of the metal from the protecting group, allowing conjugation to occur, but (most likely) without ECL. A better way to facilitate the reaction would be to use a buffer that would dissolve ruthenium more easily without affecting the protecting group. Any buffer can be used so long as it does not contain amines (such as tris), as a buffer with amines would compete with the ruthenium protecting group for conjugation with biotin. Additionally, if biotin were exposed to too much air, the moisture could have dissolved it, preventing any reactions from occurring with biotin. Minimizing moisture exposure to biotin before the reaction would allow for effective biotin-ruthenium conjugate to form to be used in further ECL studies. 25 Once the conjugation is successful, the biotin ruthenium conjugation can be used as a label for the desired molecule of detection. One such way to use biotin and ruthenium for targeting cellular compounds is to encapsulate the conjugate into liposomes, which can permeate cell membranes much more easily than pure conjugates13. Additionally, liposomes can be used as models of cell membranes13,14. Biotin-ruthenium conjugates labeled with DNA or RNA can bind to the DNA or RNA of a desired sample sequence to detect the presence or absence of a gene in an organism, or even an organism itself15. Future work is going to involve encapsulation of the conjugate into liposomes, and labeling with DNA and mRNA of organisms for detection purposes. 26 REFERENCES 1. Pyati, R., Richter, M.M. ECL-Electrochemical Luminescence. Annu. Rep. Prog. Chem. Section C, 103, 2007. pp. 12-78. 2. Blackburn, G.F., Shah, H.P., Kenton J.H., et. al. Rapid Electrochemiluminescence Assays of Polymerase Chain Reaction Products. Clinical Chemistry, 37, 1991. pp. 1626-1632. 3. Knight, A.W., Greenway, G.M. Relationship Between Structural Attributes and Observed Electrogenerated Chemiluminescence (ECL) Activity of Tertiary Amines as Potential Analytes for the tris(2,2’-bipyridyl) ruthenium (II) ECL Reaction. Analyst, 121, 1996. pp. 101R-106R. 4. Fang, L., Lü, Z., Wei, H., et. al. Quantitative Electrochemiluminescence Detection of Proteins: Avidin-Based Sensor and tris(2,2’-bipyridine) ruthenium (II) Label. Biosensors and Bioelectronics, 23, 2008. pp. 1645-1651. 5. Miao, W., Bard, A.J. Electrogenerated Chemiluminescence. 72. Determination of Immobilized DNA and C-Reactive Protein on Au (111) Electrodes Using Tris(2,2’-bipyridyl) Ruthenium (II) labels. Analytical Chemistry, 75, 2003. pp. 5825-5834. 6. Bragg, P.D., Hou, C. Subunit Composition, Function, and Spatial Arrangement, in Ca2+-Activated and Mg2+-Activated Adenosine Triphosphatases of Escherichia coli and Salmonella typhimurium. Arch. Biochem. Biophys., 167, 1975. pp. 311-321. 7. Staffilani, M., Hǒss, E., Giesen, U., et. al. Multimetallic Ruthenium(II) Complexes as Electrochemiluminescent Labels. Inorganic Chemistry, 42, 2003. pp. 7789-7798. 27 8. Slim, M., Sleiman, H.F. Ruthenium (II)-Phenanthroline-Biotin Complexes: Synthesis and Luminescence Enhancement Upon Binding to Avidin. Bioconjugate Chemistry, 15, 2004. pp. 949-953. 9. Lassman, M.E., Kulagina, N., Taitt, C.R. Fragmentation of Biotinylated Cyclic Peptides. Rapid Communications in Mass Spectrometry, 18, 2004. pp. 12771285. 10. Roesli, C. Neri, D., Ryback, J. In vivo Protein biotinylation and Sample Preparation for the Proteomic Identification of Organ- and Disease- Specific Antigens Accessible from the Vasculature. Natural Protocols, 1, 2006. pp. 192199. 11. Rybak, J.N. Scheurer, S.B., Neri, D., et. al. Purification of Biotinylated Proteins on Streptavidin Resin: A Protocol for Quantitative Elution. Proteomics, 4, 2004. pp. 2296-2299. 12. Soller, T., Ringler, M., Wunderlich, M., et. al. Streptavidin Reduces Oxygen Quenching of Biotinylated Ruthenium(II) and Palladium(II) Complexes. Journal of Physical Chemistry, 112, 2008. pp. 12824-12826. 13. Zhan, W. and Bard, A.J. Electrogenerated Chemiluminescence. 83. Immunoassay of Human C-Reactive Protein by Using Ru(bpy3)2+Encapsulated Liposomes as Labels. Analytical Chemistry, 79, 2007. pp. 459463. 14. Zhan, W. and Bard, A.J. Scanning Electrochemical Microscopy. 56. Probing Outside and Inside Single Giant Liposomes Containing Ru(bpy3)2+. Analytical Chemistry, 76, 2006. pp. 726-733. 15. Svensson, F.R., Li, M., Nordén, B., et. al. Luminescent DipyridophenazineRuthenium Probes for Liposome Membranes. Journal of Physical Chemistry B, 112, 2008. pp. 10969-10975. 28 CHAPTER 3 Long-Term Stability of Hydrophobicity and Carboxyl Functionality on Polystyrene Surfaces 3.1: Introduction 3.1.1: Polystyrene: Design of Microfluidic Devices Polystyrene is a polymer characterized by an alkyl chain with every other carbon containing a benzyl ring attached to it. Polystyrene replaced glass in the 1960s to study the interactions between cells and surfaces. It was cheap, easy to manufacture, and cell cultures would easily adsorb onto the surface when modified1. Modification of polystyrene surfaces allows for a cheap, disposable way to observe cell interactions2. Polystyrene has been employed with the used of assays for detection of biological molecules, even such that detection can occur at low concentrations of the molecule3. 3.1.2: Stability of UV and Ozone Treated Surfaces The lack of charge in polystyrene makes it a very hydrophobic, nonconducting surface. Independently, UV and ozonolysis5 have been used to make polystyrene more hydrophilic. Additionally, other reactions have been used to make polystyrene surfaces more hydrophilic6,7,8. Research has shown that coupling UV light and ozone functionalizes the surface with carboxylic acid more efficiently than UV or ozone independently1. More specifically, this surface coverage is more uniform and consistent than using UV light and ozone separately1. 29 Following UV and ozone exposure, polystyrene surfaces become functionalized with carbonyl, alcohol, ether, and ester groups in addition to the carboxylic acid functionality1. These functional groups can also affect the hydrophobicity of polystyrene, while not altering the surface charge. It has been shown that following UV and ozone treatment, polystyrene surface functionality has been maintained for a long period of time after treatment1. Longer exposure to UV and ozone is effective at functionalizing the surface, but ultimately unnecessary as carboxylic acid surface concentration reaches a maximum after ten minutes of exposure1. Additionally, while the surface chemistry of polystyrene is not altered eight months after exposure, it has not been determined at what point after treatment did polystyrene reach a saturation concentration of carboxylic acids. It is also unknown under what storage conditions may polystyrene is maintained to achieve optimum surface concentration. In this experiment, polystyrene was UV-treated with ozone gas flow to generate a more hydrophobic surface and increase the carboxyl surface concentration. The hydrophobicity and carboxyl surface concentration was characterized by measuring water contact angles and toluidine blue O (TBO) Assays. Long-term stability of hydrophobicity and carboxyl surface chemistry was compared for samples stored in phosphate buffer against samples stored in dry conditions. There was also a comparison of samples stored at room temperature, refrigeration temperature, and freezer temperature. Samples were tested for water contact angle and carboxyl surface concentration by comparing samples that reached equilibrium surface concentrations stored under different buffers (see chapter 4). 30 3.2: Materials and Methods 3.2.1: Sample Preparation Polystyrene samples were stored at three different temperatures (23oC, 4oC, and 20oC), in two different storage mediums (dry vacuum sealed bags and 0.1 M phosphate buffer, pH 7.0), at nine different time points following treatment (1 hour, 5 hours, 24 hours, 48 hours, 4 days, 7 days, 2 weeks, 3 weeks and 4 weeks). Polystyrene samples were cut from Petri dish plates. Samples were roughly 10 cm2 in area. Eight pieces were cut for each condition tested. After shaping the samples, the samples were cleaned via sonication in 50% isopropanol in water at room temperature for ten minutes. Samples were then rinsed with deionized water and dried with N2. Most of the dried samples were placed under ultraviolet (UV) light with O3 flown in at 0.5 L/min for ten minutes, followed by a two minute flush with air. Light was applied using a Jelight UVO-Cleaner, model# 144AX, at a wavelength of 254 nm using a power of 28 mW/cm2. Light was emitted ~1 cm above the tray surface. Most of these samples were washed with deionized water and dried with N2.Samples that needed to be stored were stored immediately after the second drying with N2 gas. Additional samples tested were polystyrene that was not treated with UV light and ozone, polystyrene that was treated with both UV light and ozone but that was not washed after treatment, and polystyrene that was washed after treatment, but not stored in any medium (immediate testing after last cleaning). 3.2.2: Water Contact Angle Analysis For each condition, four samples were used for water contact angle analysis. Samples stored in phosphate buffer were rinsed with deionized water and dried with N2 before 31 contact angles were measured. Five 3 µL droplets were placed on each slide. Droplets were examined using an LW Scientific Inc. Minivid. Windows Movie Maker was used to record images of droplets, while the LBASDA plugin to Image-J from the École Polytechnique Fédéral de Lausanne was used to analyze the contact angles. Water contact angles were measured using Thomas Young’s equation for surface wetting: (2) γl,g cosθ = γl,g – γs,l Where g, l, and s represent the gas, liquid and solid phases, θ is the water contact angle, and γ is the excess free energy per unit area at the interface9. This equation was used to assist in the approximation of the water contact angles with the polystyrene surface. The program utilizes a spline approach, with set boundary conditions for control points of the droplet surface and assumes that the reflection of the droplet can be seen under the glass surface10. The program’s standard error is within three degrees of the water contact angle. 3.2.3: Toluidine Blue O (TBO) Assay for Quantifying Carboxyl Surface Concentration Four samples for each condition (storage temperature, storage medium, and storage time) were used in the analysis of carboxyl surface functionality. TBO was diluted to a concentration of 500 µM in pH 10 water for binding to carboxyl functional groups. Sample surfaces were place in contact with the TBO solution for two hours. After two hours, each sample was washed three times with pH 10 water to remove any dye that 32 TBO Calibration 4.5 4 y = 3042.8x 0.7834 R2 = 0.9905 Absorbance 3.5 3 2.5 2 1.5 1 0.5 0 0 0.00005 0.0001 0.00015 0.0002 0.00025 Concentration (M) Figure 3.1 Calibration curve for determination of carboxyl surface concentration on UV treated polystyrene surfaces. It is assumed that the number of moles in the dye is equal to the number of moles of carboxylic acid functional groups on the surface of the polystyrene. Surface concentration calculation was performed by multiplying the dye concentration by the desorbing volume and dividing over the desorbing area. did not adsorb onto the surface. Samples were left to air dry for one hour. After surfaces were dried, areas of approximately 3.4 cm2 were desorbed from each sample using 50% wt acetic acid. Desorbing area was outlined by polydimethylsiloxane (PDMS) wells. These wells were cut to contain a base area of about 3.4 cm2. PDMS sides were cleaned with scotch tape between uses, and stuck to the surface to be desorbed. Desorption lasted about fifteen minutes. Desorbed samples were measured for absorbance at a wavelength of 633 nm using a spectrophotometer. A standard curve was generated using concentrations of 5 µM, 10 µM, 20 µM, 50 µM, 75 µM, 100 µM, 150 µM, and 200 µM (see figure 3.1). Calculation of surface concentration was performed using the following equation, fitting the curve to a power plot: 33 (3) A = k*cn A is the absorbance, k and n are constants, and c is the concentration of TBO in the solution. 3.3: Results and Discussion 3.3.1: Analysis of Controls Polystyrene surfaces were successfully functionalized as a result of the UV and ozone treatment. The average water contact angle for a sample that was not treated with UVlight and ozone was 82.0o with a standard deviation of about 6.6o (see figure 3.2). Samples that were treated with UV-light and ozone, but that had not been washed and dried after treatment averaged a water contact angle of 11.0o with a standard deviation of 3.3o. The samples that were treated with UV-light and ozone, and that were washed after treatment, but not stored in any medium (zero time) averaged a water contact angle of 42.0o with a standard deviation of 7.7o. These results show that hydrophobicity significantly decreased as a result of the UV-treatment and ozone, but that many of the surface functional groups had been modified or washed off by the wetting of the surface. This is consistent with the results seen in other research11. Comparison of the non-UV treated samples to the UV-treated samples shows a dramatic increase in the functionalization of carboxyl groups on the polystyrene surface (see figure 3.3) Average carboxyl surface concentration for non-UV treated polystyrene was approximately 0.3 nmol/cm2 with a standard deviation of 46 pmol/cm2. This is significantly less than the UV and ozone treated samples that were not rinsed and the UV and ozone treated samples that were rinsed and not stored. 34 Water Contact Angle (Degrees) Water Contact Angles for Polystyrene Samples 100 90 80 70 60 50 40 30 20 10 0 No UV No Wash Not Stored Treatment Figure 3.2 Average water contact angles for non-stored polystyrene. Samples tested included polystyrene not treated with UV and ozone, polystyrene that had been treated for ten minutes, but had not been rinsed, and samples that had been rinsed after washing and were immediately tested for hydrophobicity. Carboxyl surface concentration for treated and unwashed samples averaged 15.8 nmol/cm2 with a standard deviation of 0.23 nmol/cm2. Carboxyl surface concentration for treated, washed, but not stored samples averaged 12.4 nmol/cm2 with a standard deviation of 1.24 nmol/cm2. 3.3.2: Forty Eight Hours After Initial Treatment Average water contact angles were calculated for each storage condition measured (time after treatment, storage temperature, and storage medium; see figure 3.4, and table 3.1). Overall, hydrophobic rebound occurred most rapidly in the first hour after treatment, leveling off after 24 hours of treatment. Water contact angles spiked from 42o to around 55o after the first day, with a slight decrease towards the second day. Treatment temperature appeared not affect the water contact angle significantly during the short time after UV-treatment with ozone. For both the phosphate stored samples 35 Surface Concentration (nmol/cm^2) Carboxyl Surface Chemistry 16 14 12 10 8 6 4 2 0 No UV No Wash Not Stored Surface Treatment Figure 3.3 Average carboxylic acid surface concentration for non-stored polystyrene. Samples tested included polystyrene not treated with UV and ozone, polystyrene that had been treated for ten minutes, but had not been rinsed, and samples that had been rinsed after washing and were immediately tested for hydrophobicity. and the dry stored samples, hydrophobic rebound within the hour after treatment was slowest for the 23oC samples, but by the fifth hour, the variation amongst the temperatures had settled. Comparison of the samples stored under dry conditions to the samples stored in phosphate buffer shows that there is not a significant difference in hydrophobic rebound between both storage media at any of the observed temperatures. Water Contact Angle at Various Temperatures Water Contact Angle (Degrees) Water Contact Angle (Degrees) Water Contact Angles at Various Storage Temperatures 70 60 50 23 40 4 30 -20 20 10 0 70 60 50 23 40 4 30 -20 20 10 0 0 1 5 24 48 0 Time After UV-Treatment (Hours) 1 5 24 48 Time After UV-Treatment (Hours) Figure 3.4 Average water contact angle for polystyrene 1 hr, 5 hr, 24 hr, and 48 hr after UV and ozone treatment. Blue bars represent samples stored at 23oC, red bars represent samples stored at 4oC and green bars represent samples stored at -20oC. The graph on the left depicts samples stored in dry conditions while the graph on the right represents samples stored in 0.1 M pH 7 sodium phosphate buffer. 36 Carboxyl Surface Chemistry Surface Concentration (nmol/cm^2) Surface Concentration (nmol/cm^2) Carboxyl Surface Chemistry 18 16 14 12 23 10 4 8 -20 6 4 2 16 14 12 10 23 8 4 6 -20 4 2 0 0 0 1 5 24 0 48 1 5 24 48 Time after Treatment (Hours) Time After Treatment (Hours) Figure 3.5 Average carboxyl surface concentration for polystyrene 1 hr, 5 hr, 24 hr, and 48 hr after UV and ozone treatment. Blue bars represent samples stored at 23oC, red bars represent samples stored at 4oC and green bars represent samples stored at -20oC. The graph on the left depicts samples stored in dry conditions while the graph on the right represents samples stored in 0.1 M pH 7 sodium phosphate buffer. Carboxyl surface concentration appears to decrease rapidly within the first hour for polystyrene stored in phosphate buffer (see figure 3.5 and table 3.2). This dramatic reduction was seen for all of the temperatures studied. In contrast, carboxyl surface concentration for dry stored samples was greater for the 5 hr time point than it was right after UV and ozone treatment. This disparity seen between the one hour and five hour can be attributed to the leakiness of the PDMS wells used to confine the desorbing area. Regardless, dry stored samples consistently maintained a greater carboxyl surface concentration than the polystyrene stored in phosphate buffer. Additionally, the -20oC samples stored in phosphate buffer had less of a reduction in carboxyl surface chemistry than the samples stored in the same medium but at warmer temperatures. This was seen at all time points within the first two days except one hour after treatment. However, the variation in the carboxyl surface concentration is relatively high, so the difference is ultimately not that great between the samples stored at different temperatures. 3.3.3: Seven Days After Initial Treatment Water contact angle over the first week appeared to have reached a maximum around day 4 for the dry stored samples, while the maximum angle was reached after 24 hours 37 Water Contact Angle at Various Storage Temperatures Water Contact Angle (Degrees) Water Contact Angle (Degrees) Water Contact Angles at Various Storage Temperatures 70 60 50 23 40 4 30 -20 20 10 0 70 60 50 23 40 4 30 -20 20 10 0 0 1 2 3 4 5 6 7 8 0 Time After UV-Treatment (Days) 1 2 3 4 5 6 7 8 Time After UV-Treatment (Days) Figure 3.6 Average water contact angle for polystyrene 24 hr, 48 hr, 4 days, and 7 days after UV and ozone treatment. Blue lines represent samples stored at 23oC, red lines represent samples stored at 4oC and green lines represent samples stored at -20oC. The graph on the left depicts samples stored in dry conditions while the graph on the right represents samples stored in 0.1 M pH 7 sodium phosphate buffer. of storage for the phosphate stored samples (see figure 3.6 and table 3.1). Most of the water contact angles measured averaged between 50 and 55o, with none falling outside the range of 47-60o. This indicates an overall hydrophobic rebound from the original measurement, but with leveling off occurring significantly less than that of polystyrene that was not exposed to UV-light and ozone simultaneously. Comparing temperatures shows that contact angles were greatest for the 23oC stored samples, with a slightly less contact angle for the 4oC and -20oC stored samples, though comparison of the two cooler temperatures shows little difference in the contact angle. The exception to this occurred four days after treatment for the dry stored samples and 24 hours after treatment for the phosphate stored samples. Comparison of the dry stored samples to the phosphate stored samples during the first week after treatment showed little variation between samples. Additionally, water contact angle appeared to be decreasing by end of the first week, approaching a value of around 50o. Looking at samples after one week of treatment shows a leveling off of carboxyl surface concentration for polystyrene stored at all temperatures and in both mediums (see figure 3.7 and table 3.2). Dry stored samples appeared to have leveled off at 17 nmol/cm2, while -20oC samples stored in pH 7.0 phosphate buffer maintained a surface concentration of approximately 2 nmol/cm2. Additionally, surface 38 Carboxyl Surface Chemistry Surface Concentration (nmol/cm^2) Surface Concentration (nmol/cm^2) Carboxyl Surface Chemistry 20 18 16 14 12 23 10 4 8 -20 6 4 2 0 16 14 12 10 23 8 4 6 -20 4 2 0 0 1 2 3 4 5 6 7 8 0 Time After Treatment (Days) 1 2 3 4 5 6 7 8 Time After Treatment (Days) Figure 3.7 Average carboxyl surface concentration for polystyrene 24 hr, 48 hr, 4 days, and 7 days after UV and ozone treatment. Blue lines represent samples stored at 23oC, red lines represent samples stored at 4oC and green lines represent samples stored at -20oC. The graph on the left depicts samples stored in dry conditions while the graph on the right represents samples stored in 0.1 M pH 7 sodium phosphate buffer. concentrations of samples stored at 4oC and 23oC at room temperature averaged a surface concentration of less than 1 nmol/cm2. This is consistent with the results of the first 48 hours, showing that samples stored in dry, vacuum-sealed bags maintain a higher carboxyl surface concentration than samples stored in phosphate buffer. Additionally, the higher concentration with the lower temperature in the phosphate samples is more pronounced during this duration after treatment. The variation between surface concentrations is less with these samples than it was with the one hour and 5 hour samples. Thus, -20oC storage seems to have more favorable shortterm maintenance of carboxyl surface functionality than either the refrigerated or room-temperature stored samples. 3.3.4: Four Weeks After Initial Treatment Overall, there was an increase in the measured water-contact angle over the four week period after UV and ozone treatment (see figure 3.8 and table 3.1). However, hydrophobic rebound mostly leveled off after the second week following measurements. Comparison of the samples of similar temperatures shows greater variation amongst the dry stored samples. Specifically, the samples stored at higher 39 Water Contact Angle At Various Storage Temperatures 70 Water Contact Angle (Degrees) Water Contact Angle (Degrees) Water Contact Angle at Various Storage Temperatures 60 50 23 40 4 30 -20 20 10 0 70 60 50 23 40 4 30 -20 20 10 0 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 0 0.5 1 Time After UV-Treatment (Weeks) 1.5 2 2.5 3 3.5 4 4.5 Time After UV-Treatment (Weeks) Figure 3.8 Average water contact angle for polystyrene 7 days, 14 days, 3 weeks, and 4 weeks after UV and ozone treatment. Blue lines represent samples stored at 23oC, red lines represent samples stored at 4oC and green lines represent samples stored at -20oC. The graph on the left depicts samples stored in dry conditions while the graph on the right represents samples stored in 0.1 M pH 7 sodium phosphate buffer. temperatures appeared to have greater hydrophobic rebound. However, all the measurements still showed that the average contact angle was significantly less than that of the non-UV treated sample; none of the average water contact angles measured greater than 60o. For the phosphate stored samples, variation was less in the water contact angles measured. The values measured were similar to that of the water contact angles measured for the 4oC and the -20oC samples stored under dry conditions. Overall, hydrophobic rebound remains stable weeks after UV and ozone treatment for both storage media and at all the temperatures (though lower temperatures appear to be more preferable than higher temperatures for maintaining lower hydrophobicity). Carboxyl Surface Chemistry Surface Concentration (nmol/cm^2) Surface Concentration (nmol/cm^2) Carboxyl Surface Chemistry 20 18 16 14 12 23 10 4 8 -20 6 4 2 0 16 14 12 10 23 8 4 6 -20 4 2 0 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 0 Time After Treatment (weeks) 0.5 1 1.5 2 2.5 3 3.5 4 4.5 Time After Treatment (weeks) Figure 3.9 Average carboxyl surface concentration for polystyrene 7 days, 14 days, 3 weeks, and 4 weeks after UV and ozone treatment. Blue lines represent samples stored at 23oC, red lines represent samples stored at 4oC and green lines represent samples stored at -20oC. The graph on the left depicts samples stored in dry conditions while the graph on the right represents samples stored in 0.1 M pH 7 sodium phosphate buffer. 40 Looking up to four weeks of storage, it can be seen that a greater surface concentration is maintained in the dry stored samples than in the phosphate stored samples (see figure 3.9 and table 3.2). Samples at the two and three week time points appear to have a lower carboxyl surface concentration than those one week and four weeks after treatment. This variation is likely due to the leakiness of the PDMS wells. The opposite was seen with the phosphate stored samples, where the concentration increased into the second week samples, but leveled off after the third week. The 20oC polystyrene samples stored in phosphate buffer had greater carboxyl surface concentration than the other samples stored in the phosphate buffer until the fourth week, which saw a huge drop in the surface concentration of the frozen samples. Until this time point, -20oC stored samples had a higher carboxyl surface concentration than the room temperature stored and refrigerated polystyrene samples. Conclusions and Future Work The results of the TBO Assay and water contact angle analysis show that carboxyl surface functionality can be well maintained weeks after treatment with UV-light and ozone. It also shows that surface saturation for both carboxylic acid functionality and water contact angle can occur within as little as one hour after treatment. Additionally, temperature of storage seems to have little effect on the water contact angle of samples stored in both mediums. This shows that if a surface conjugation needed to occur between 23oC and -20oC, it could without the reduced functionality of the device. The results also show that samples stored in a dry medium provide better storage for UV and ozone treated polystyrene than samples stored in 0.1 M pH 7 sodium phosphate buffer. While water contact angle is maintained, carboxylic acid surface 41 concentration decreases. Further more, samples stored in phosphate buffer are also more temperature sensitive than samples stored in water, as samples stored at -20oC maintained some carboxyl surface functionality, while virtually none of the room temperature or refrigerated samples had carboxyl groups on them past the first day. Dry stored samples showed no consistency as to when the surface concentration or the water contact angle was greatest for a given temperature. Future experiments involve other modifications that may be done with storage. When observing samples stored in sodium phosphate buffer, there may be a specific timing as to when saturation is reached, and how rapidly the surface concentration drops off. Additionally, dry stored samples can be tested to see if moisture sensitivity can affect the hydrophobicity and carboxyl surface concentration of UV treated polystyrene. 42 Table 3.1 Average water contact angles for UV and ozone treated polystyrene samples stored over a four week duration. Average Standard Dry Stored Deviation Average Phosphate Buffer Stored Standard Deviation Average Standard Dry Stored Deviation Average Phosphate Buffer Stored Standard Deviation Temp 1 hr 5 hrs 24 hrs 48 hrs 4 days 49.9 52.4 53.0 55.4 23 57.4 54.7 53.2 47.3 50.8 4 59.2 52.1 51.5 48.3 50.3 -20 54.8 6.0 4.9 5.3 3.5 23 3.8 3.8 4.1 6.2 4.1 4 5.2 2.4 5.1 7.2 6.0 -20 3.4 50.7 51.3 57.3 55.9 23 54.4 52.7 52.1 58.9 52.0 4 52.8 51.7 54.5 57.1 52.5 -20 50.8 2.2 4.2 3.8 4.6 23 3.3 2.1 3.7 2.9 4.6 4 4.4 2.7 4.9 4.5 2.8 -20 4.0 Temp 7 days 2 wks 3 wks 4 wks 54.2 58.5 56.8 58.9 23 49.5 57.1 54.9 55.0 4 50.8 51.5 52.6 52.5 -20 4.9 5.1 5.2 4.4 23 4.6 3.4 6.9 2.9 4 3.0 4.0 3.7 3.0 -20 51.5 55.3 54.9 56.5 23 50.5 55.2 56.9 59.5 4 50.1 53.8 57.7 59.0 -20 7.2 4.0 5.7 3.6 23 3.4 4.4 4.7 3.5 4 3.6 3.1 4.6 3.6 -20 43 Table 3.2 Average carboxyl surface concentration for UV and ozone treated polystyrene samples stored over a four week duration. Average Dry Stored Standard Deviation Average Phosphate Buffer Stored Standard Deviation Average Dry Stored Standard Deviation Average Phosphate Buffer Stored Standard Deviation Temp 1 hr 5 hr 24 hr 48 hr 4 days 5.9 15.8 9.2 10.7 17.4 23 7.1 15.6 8.9 11.1 17.1 4 4.0 15.6 7.5 9.2 16.3 -20 2.22 0.20 2.24 1.45 0.11 23 1.99 0.40 0.35 1.84 0.38 4 1.34 0.54 1.32 2.23 0.75 -20 2.6 5.6 2.0 1.6 0.3 23 1.7 5.8 2.1 2.1 0.2 4 2.1 8.2 2.2 2.2 1.7 -20 0.87 1.50 0.20 0.13 0.05 23 0.57 0.82 0.07 0.26 0.01 4 0.13 1.10 0.73 0.30 0.51 -20 Temp 7 days 2 wks 3 wks 4 wks 16.9 13.5 13.5 17.2 23 17.0 11.8 12.3 17.7 4 17.0 13.3 13.0 17.4 -20 0.34 1.42 0.42 0.38 23 0.15 0.99 1.24 0.31 4 0.09 0.49 0.96 0.27 -20 0.2 5.2 4.1 4.8 23 0.2 5.7 5.5 5.2 4 1.7 7.1 6.1 0.4 -20 0.03 0.93 0.66 0.72 23 0.02 0.56 0.28 0.38 4 0.35 0.62 0.02 0.19 -20 44 REFERENCES 1. Callen, B.W., Ridge, M.L. Lahooti, S. Remote Plasma and Ultraviolet-Ozone Modification of Polystyrene. J. Vac. Sci. Tehnol. A., 13, 4, 1995. pp. 20232029. 2. Curtis, A.S.G., Forrester, J.V., McInnes, C., et. al. Adhesion of Cells to Polystyrene Surfaces. J. Cell Biol., 97, 1983. 1500-1506. 3. Do, J., Ahn, C.H. A Polymer Lab-on-a-Chip for Magnetic Immunoassay with On-Chip Sampling and Detection Capabilities. Lab Chip, 8, 2008. pp. 542549. 4. Bliznyuk, V., Assender, H., Briggs, A., et. al. Surface Glass Transition Temperature of Amorphous Polystyrene Measured by SFM. APS Meeting, March 2001. 5. Jellinek, H.H.G. and Kryman, F.J. Gas Analysis by Polymer Chain Scission: Ozonolysis of Polystyrene. Environmental Science and Technology, 1, 1967. pp. 658-660 6. Callen, B.W., Sodhi, R.N.S., Shelton, R.M., et. al. Behavior of Bone Cells Characterized on Polystyrene Surfaces. Journal of Biomedical Materials Research, 27, 1993. pp. 851-859 7. Curtis, A.S.G., Forrester, J.V., and Clark, P. Substrate Hydroxylation and Cell Adhesion. Journal of Cell Science, 86, 1986. pp. 9-25. 8. Ertel, S.I., Chilkoti, A., Horbett, T.A., et. al. Endothelial Cell Growth on Oxygen-Containing Films Deposited by Radio-Frequency Plasmas: the Role of Surface Carbonyl Groups. Journal of Biomaterials Science: Polymer Edition, 3, 1991. pp. 163-183. 9. T. Young. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond., 1905. pp. 65-87. 45 10. Stalder, A.F. Kulik, G., Sage, D. A Snake-Based Approach to Accurate Determination of Both Contact Points and Contact Angles. Colloids and Surfaces A: Physicochem. Eng. Aspects, 286, 2006. pp. 92-103. 11. Zhang, D., Dougal, S.M., and Yeganeh, M.S. Effects of UV Irradiation and Plasma Treatment on a Polystyrene Surface Studied by IR-Visible Sum Frequency Generation Spectroscopy. Langmuir, 16, 2000. pp. 4528-4532. 46 CHAPTER 4 The Affects of Heating, Surface Coating, and Buffer Storage on Hydrophobicity and Carboxyl Functionality of UV-Treated Polystyrene and PMMA Surfaces 4.1: Introduction 4.1.1: PMMA: Design of a Microfluidic Device Poly(methyl methacrylate) (PMMA) is an alkyl polymer characterized by which every other carbon forming methyl acetate group on the surface. These repeating ester groups within PMMA allow for it to have some reactivity, while maintaining a mostly stable structure. It has been shown that PMMA conjugated with graphite can be more electrically conductive, and allow for efficient transport of solutes formed in channels of microfluidic devices made of PMMA1. Additionally, PMMA is used because of its transparency and low autofluorescence2. Like polystyrene, PMMA is a very hydrophobic material before UV-treatment. After UV treatment with ozone, the PMMA surface polymer becomes replaced with alcohol, ether, ester, carbonyl, and carboxyl groups. PMMA be used effectively in the development of microfluidic devices because of its good surface bonding at low temperatures3. Additionally, PMMA is cheap and easy to make, which has allowed it to replace glass in the formation of many devices. Due to the good conjugation chemistry between carboxylic acids and many biomolecules, assays can be coupled with the microfluidics, forming devices that can serve multiple functions4. 47 4.1.2: Thermal and Chemical Effects on Carboxyl Surface Chemistry and Hydrophobicity In the formation of microfluidic devices, polystyrene or PMMA may be heated to facilitate the creation of the channels in the device, or fort bonding purposes5. This can be done as an alternative to formation of channels using polydimethylsiloxane (PDMS), which is better suited for the formation of channels requiring hydrophobic small molecule attachment or conjugation6. While polystyrene has a melting temperature of approximately 240oC5, PMMA has a melting temperature of ~130135oC7. While heating for channel formation usually occurs at 80-100oC, polystyrene glass can warp or dissolve around 95oC5.8. Conversely, the glass melting temperature (or glass transition temperature) of PMMA is approximately equal to its melting temperature9. Additionally, any heat applied to polystyrene in the presence of water can distort the shape of the device, and ultimately impair the ability of the channels to form properly. However, altering the glass transition temperature has been shown to allow for bonding between surfaces without altering the geometry of the channel10. Glass melting temperatures may also vary depending on the thickness of the device and the presence of other materials within the compound (other plastics, impurities, etc)11. Surface wetting can often be used to facilitate the bonding of two surfaces of a device12. After polystyrene is treated with UV light and ozone, the surface will be bound to another surface, forming the device. This would entail the surface being coated with acetone or acetyl acetone, followed by immediate binding between the treated surface and the other surface to be bound13. Additionally, acetyl acetone can 48 form cyclic compounds with many metals, which can promote the formation of electrodes onto a PMMA or polystyrene surface14. While the development of microfluidic devices using polystyrene is well known, it is unknown if any of these steps effect the hydrophobicity and carboxyl surface functionality of PMMA or polystyrene. It is important to know if these factors affect surface chemistry as it could impact the methods of developing devices with carboxylic acids functionalized on the surface. Additionally, while surface chemistry has been shown to affect glass transition temperature15, it is unknown whether or not glass transition temperature can affect surface chemistry, specifically, if it affects carboxylic acid surface chemistry. In this experiment, several factors affecting carboxyl surface concentration and hydrophobicity were compared. First, UV and ozone treated PMMA and polystyrene were heated to three different temperatures and tested to see if the heating reduced the carboxyl surface concentration. Next, polystyrene was coated with a surface bonding solvent (acetyl acetone) to determine if that also reduced carboxyl surface concentration. Last, as an extension of the research described in chapter 3, samples were stored in different buffers and at different pH for a short amount of time to determine if carboxyl surface concentration was reduced, and if hydrophobicity increased. These results would ultimately show the optimum storage conditions for UV-treated polystyrene, the best conjugation buffer for any biomolecule to be immobilized to the polystyrene surface, and how factors used for surface bonding would affect the surface functionality and hydrophobicity. 49 4.2: Materials and Methods 4.2.1: Sample Preparation Eight samples were prepared for each condition observed. Conditions tested included a comparison of PMMA to Polystyrene, and three temperatures (90oC, 95oC, and 100oC) used in the formation of channels in microfluidic devices. PMMA samples were cut to 2 cm by 3 cm samples. Polystyrene samples were cut as described in chapter 4. Three control sample types were also prepared: non-UV treated samples, samples that had been treated with UV and ozone, but had not been washed, and samples that had been washed but without heating occurring. Samples were cleaned via ten minute sonication in 50% isopropanol in water at room temperature. Samples were then rinsed with deionized water and dried with N2 gas. Samples were exposed to UV light and ozone for ten minutes with an ozone flow rate of 0.5 L/min. This was followed by a two minute flush. Samples to be used for temperature experiments were heated to the desired temperature on a (insert name of machine here) for five minutes. Following the heating, samples were washed with deionized water and dried with N2 gas to be used for experiments. Spin coated samples were treated with UV and ozone as described in chapter 4. After the second wash and dry with deionized H2O and N2, polystyrene samples (UV treated and non-UV treated were coated) with acetyl acetone using a CHEMAT Technology Spin coater KW-4A. Droplets were placed on the polystyrene surface and were spun at 1000 rpm for 4.5 seconds. Samples stored in solution buffers were placed in five different buffers and at basic, neutral, or acidic pH for each buffer. Samples stored in water or in 0.1 M PBS were 50 tested for water contact angle and hydrophobicity at pH 5, 7, and 9. Samples stored in 0.1 M HEPES, 0.1 M Sodium Borate, or 1 M Tris were stored at pH 6, 7, and 8. Buffer and pH samples were stored for 1 hour at 23oC following treatment before being tested for water contact angle and surface carboxylic acid concentration. 4.2.2: Water Contact Angle Analysis Water Contact Angles were analyzed as shown in Chapter 3: Long-Term Stability of Hydrophobicity and Carboxyl Functionality on Polystyrene Surfaces. Four samples with five droplets per sample were used for each condition (channel material and temperature of heating). 4.2.3: TBO Assay for Quantified Carboxyl Surface Concentration TBO Analysis was performed as explained in Chapter 3: Long-Term Stability of Hydrophobicity and Carboxyl Functionality on Polystyrene Surfaces. The only modification made to the analysis was for the PMMA surfaces, the desorbing area covered the entire 6 cm2 sample. Consequentially, surface concentration had to change based on that model. 4.3: Results and Discussion 4.3.1: PMMA and Polystyrene: Thermal Bonding Like polystyrene, PMMA shows a similar trend in comparing non-UV treated ozone to UV-treated ozone (see figure 4.1) However, the extremity of the values was much more significant for the polystyrene samples than the PMMA samples. PMMA that 51 was not treated with UV averaged a water contact angle of 66.0o with a standard deviation of 3.6o. PMMA that was treated with UV and ozone but that had not been rinsed following treatment average a water contact angle of 28.4o with a standard deviation of 3.4o. While these PMMA samples showed less variation in contact angles, the reduction in hydrophobicity following UV treatment was significantly less than that seen in polystyrene. Furthermore, samples that had been rinsed with water after treatment without heating experienced an average water contact angle of 36.2o with a standard deviation of 4.1o. This value is significantly less that of rinsed, UV-treated polystyrene. Additionally, the rebound that occurs due to washing is much less than that of polystyrene. Washing shows an average increase in water contact angle of 7.8o for PMMA and 31.0o for polystyrene. These results indicate that UV-treatment with ozone may affect the surface bonding chemistry of polystyrene more than in PMMA. Comparison of heated samples to unheated samples shows a complete hydrophobic in Water Contact Angle (Degrees) Water Contact Angles for PMMA Samples 90 80 70 60 50 40 30 20 10 0 No UV No Wash Not Heated Treatment Figure 4.1 Water contact angle analysis for PMMA control samples. Included in the control samples are untreated samples, PMMA samples treated with UV light and ozone, but not washed, and PMMA samples that were washed with deionized water following treatment, but were not heated before washing. 52 Water Contact Angle (Degrees) Water Contact Angles for Samples Heated for Five Minutes 80 70 60 50 Polystyrene 40 PMMA 30 20 10 0 No Heat 90 95 100 Temperature of Heat (degrees Celsius) Figure 4.2 Water contact angle analysis for UV and Ozone treated PMMA and polystyrene heated samples. Blue bars represent polystyrene, while red bars represent PMMA. All samples were UV and ozone treated, some of the samples were heated to 90, 95, or 100 oC for five minutes before a final rinse with deionized water. heating with PMMA (see figure 4.2 and table 4.1). For all cases of heating, average water contact angle was within range of the water contact angle of non-treated samples. The lowest contact angles measured occurred at 90oC heating, while the highest contact angles measured came from the 95oC heated samples, with the 100oC heated samples having similar contact angles to that of non-UV treated samples. In comparison, polystyrene samples experienced almost negligible hydrophobic rebound upon being heated (see figure 4.2 and table 4.1). Though all the samples did show some hydrophobic rebound after heating, the average value for each heated sample was less than that of one hour storage time for all different temperature and storage conditions. Additionally, results show that there is little effect that the heating temperature has on the water contact angle. This was true for both polymers, but even more so for polystyrene where the difference between the greatest and least of these contact angles was about 1.5o. 53 Surface Concentration (nmol/cm^2) Carboxyl Surface Chemistry, PMMA Samples 12 10 8 6 4 2 0 No UV No Wash Not Stored Surface Treatment Figure 4.3 Carboxylic acid surface quantification for PMMA control samples. Included in the control samples are untreated samples, PMMA samples treated with UV light and ozone, but not washed, and PMMA samples that were washed with deionized water following treatment, but were not heated before washing. Like polystyrene, carboxyl surface concentration increased on PMMA surfaces after applying UV light and ozone for ten minutes (see figure 4.3). However, hydrophobic rebound did not occur when the PMMA samples were washed. Comparison shows that UV-treated PMMA (washed and unwashed) had a carboxyl surface concentration of 9.8 nmol/cm2, while untreated samples had 0.098 nmol/cm2 carboxyl surface concentration. Ultimately, the UV-treatment with ozone did functionalize the surface with carboxylic acids and washing did not affect carboxylic acid functionality. Heating of polystyrene surfaces seemed to have decreased carboxyl surface concentration, while heating of PMMA surfaces did not effect carboxyl surface concentration (see figure 4.4 and table 4.2). After heating, carboxylic acid surface concentration on polystyrene appears to drop from approximately 12 nmol/cm2 to 7 nmol/cm2. However, measured surface concentrations of all PMMA surfaces following heating fell between 9.7 and 10 nmol/cm2. Thus, both surfaces will still 54 Surface Concentration (nmol/cm^2) Carboxyl Surface Chemistry, Heated Samples 16 14 12 10 Polystyrene 8 PMMA 6 4 2 0 No Heat 90 95 100 Temperature of Heating (Celsius) Figure 4.4 Carboxylic acid surface quantification for UV and Ozone treated PMMA and polystyrene heated samples. Blue bars represent polystyrene, while red bars represent PMMA. All samples were UV and ozone treated, some of the samples were heated to 90, 95, or 100 oC for five minutes before a final rinse with deionized water. retain most of the carboxyl surface functionality, with UV-treated PMMA being completely unaffected by heat. 4.3.2: Polystyrene and Solvent Bonding Comparison of spin coated and non-spin coated samples showed complete hydrophobic rebound following spin-coating (see figure 4.5). UV treated and spin coated samples had an average water contact angle of 84.9o, while non-UV-treated samples had an average water contact angle of 81.7o. Ultimately, there was no significant difference between non-UV-treated and acetyl acetone coated samples, UV-treated and acetyl acetone coated samples, and non-UV-treated samples. Samples spin coated with acetone showed a reduction in carboxyl surface functionality compared to UV-treated and washed samples (see figure 4.6). Non-UV treated acetyl acetone covered samples averaged a surface concentration of 0.22 55 Water Contact Angle (Degrees) Water Contact Angle for Acetyl Acetone Coated Samples 100 90 80 70 60 50 40 30 20 10 0 Non UV UV Treatment Figure 4.5 Water contact angle analysis for polystyrene with a surface coating of acetyl acetone. Comparison of non treated against UV and ozone treated samples. nmol/cm2 while UV-treated, acetyl acetone coated samples averaged a surface concentration of 6.5 nmol/cm2. While the error range on the untreated samples was low, UV-treated samples had a standard deviation of 4.4 nmol/cm2. Thus, there is still a greater carboxyl surface concentration for UV-treated samples. However, there is a significant reduction in the surface concentration after coating the surface with acetyl acetone as the functional groups will be covered by the solvent. The spin coated samples did not appear uniformly blue. This suggests that acetyl acetone did not completely coat the surface of the sample. This non-uniform coating may be responsible for the large variance observed by the UV-treated and acetyl acetone coated samples 4.3.3:Buffer and pH Experiments 56 Surface Concentration (nmol/crm^2) Carboxyl Surface Chemistry, Acetyl Acetone Spindown samples 12 10 8 6 4 2 0 No UV UV Treatment Figure 4.6 Carboxylic acid surface quantification for polystyrene with a surface coating of acetyl acetone. Comparison of non treated against UV and ozone treated samples For pH tested samples, H2O and tris buffer stored samples maintained the lowest water contact angles, all of which averaged between fifty three and fifty six degrees (see figure 4.7 and table 4.3). Additionally, for all samples except for water and sodium borate, water contact angle increased with pH. For sodium borate, water contact angle was greatest with the pH 7 samples. For the H2O stored samples, water contact angle did not vary much with pH, though pH 9 H2O had the lowest average water contact angle measured amongst the water stored samples. pH 9 H2O and pH 6 and 7 tris appeared to have the least hydrophobic rebound of any of the samples. Sodium borate and moderate to high pH HEPES and PBS were the buffers that caused the most hydrophobic rebound. Overall, the HEPES, sodium borate, and PBS buffers caused significantly more hydrophobic rebound than sodium phosphate stored, H2O stored, tris stored, or dry stored samples, one hour after treatment. It appears that the other buffers and storage mediums did not significantly alter water contact angle. Samples stored in various buffers indicated that acidic and neutral H2O and acidic PBS can be used for maintaining carboxyl surface chemistry best (see figure 4.8 and 57 Water Contact Angle (Degrees) Water Contact Angle, Various Buffers and pH 80 70 60 Water 50 PBS 40 HEPES 30 Sodium Borate Tris 20 10 0 5 6 7 8 9 pH Figure 4.7 Water contact angle analysis for UV and ozone treated polystyrene stored in various solutions for 1 hour at either acidic, neutral or basic pH. Water and PBS buffer stored samples were analyzed at pH 5, 7, and 9. HEPES buffer, sodium borate buffer, and tris buffer stored samples were analyzed at pH 6, 7, and 8. table 4.4). HEPES and sodium borate stored samples appeared to have cut the surface concentration in half. Samples stored in tris buffer at a pH of 7 maintained a carboxyl surface concentration significantly higher than that of samples stored in HEPES and sodium borate and that of acidic or basic tris. Basic water and neutral and acidic PBS have carboxyl surface functionality similar to that of UV-treated polystyrene stored in pH 7 tris buffer. Overall, samples stored in acid or neutral water, acidic PBS, and in dry vacuum sealed bags retain the most carboxylic acid functionality for samples to be used shortly after treatment. 4.4: Conclusions and Future Work The heating of PMMA and polystyrene for surface bonding and channel formation did affect the surface chemistry. While there appeared to be no correlation between heating temperature and water contact angle, heating of the PMMA surface did 58 18 16 14 (nmol/cm^2) Carboxyl Surface Concentration Carboxyl Surface Chemistry, Varied Buffers and pH Water 12 PBS 10 HEPES 8 Sodium Borate 6 Tris 4 2 0 5 6 7 8 9 pH Figure 4.8 Carboxylic acid surface quantification for UV and ozone treated polystyrene stored in various solutions for 1 hour at either acidic, neutral or basic pH. Water and PBS buffer stored samples were analyzed at pH 5, 7, and 9. HEPES buffer, sodium borate buffer, and tris buffer stored samples were analyzed at pH 6, 7, and 8. increase hydrophobicity. However, with carboxyl surface chemistry remaining constant for all three thermal bonding temperatures, a likely event of the heating is that the surface chemistry was altered, but that carboxyl surface chemistry remained unaffected. It is likely that other hydrophilic groups were removed from the surface, such as alcohol and ether groups. It has been shown that in polystyrene, UV and ozone treatment can form functional groups other than carboxylic acids16. On the other end of the spectrum, polystyrene did not become more hydrophobic, but it did lose its surface functionality. However, the loss of functionality was also not correlated to temperature. Additionally, the final surface concentration after heating was still greater than 50% of the original surface concentration. Though there was a dramatic reduction in the carboxyl surface concentration, the reduction should not be enough to significantly alter the ability for proteins to be adsorbed onto the surface. This is further shown through the retention in hydrophobicity. 59 Acetyl acetone is a very hydrophobic solvent. As such, it is not surprising to see that there was a great reduction in the carboxyl surface concentration, as well as an increase in water contact angle to the point of having the same hydrophobicity as untreated polystyrene. The results of the TBO assay show a large span of possible surface concentrations, indicating that nothing can be concluded about the effect of acetyl acetone coating on carboxyl surface chemistry. If there is a reduction in the surface chemistry, this may not affect surface bonding. Since surface bonding occurs immediately after coating, channels would be washed out before acetyl acetone firmly dries onto the channel surface. If this occurs, surface chemistry would not be altered within the channels if acetyl acetone is properly flushed out. Determination of spin coating with acetyl acetone immediately followed by washing with water should determine whether or not the solvent bonder would affect the channel’s surface chemistry. The presence of buffers indicates the reactivity of the carboxylic acid surface. Carboxyl surface functionality was well maintained only in samples stored in dry conditions, pH 5-7 water, and pH 5 PBS buffer. Additionally, pH 7-9 PBS buffer, pH 9 H2O, and pH 7 tris buffer maintained more than 50% functionality. Looking at hydrophobicity, samples stored in dry conditions, water, 0.1 M phosphate buffer, and tris buffer increased hydrophobicity the least. These results indicate that the best suited storage for UV and ozone treated polystyrene is under dry conditions or in pH 5-7 water. The hydrophobic rebound and loss of functionality observed in the sodium borate and HEPES buffers show that they are inadequate for polystyrene storage. Additionally, low pH seems to be favorable for retaining hydrophobicity. This makes sense, as the presence of free floating hydrogen ions would increase the surface charge 60 of the negatively charged carboxyl groups. PBS pH 5 is the buffer most suitable for chemical modification of polystyrene. More buffers should be tested for surface functionality and hydrophobicity, and the pH trend in water contact angle should also be investigated with other buffers. Table 4.1 Average water contact angles on the surface of heated and UV and ozone treated polystyrene and PMMA. No Heat 90 95 Average 100 No Heat 90 95 Standard Deviation 100 Polystyrene PMMA 42.0 36.2 47.3 59.5 45.8 69.8 47.1 66.4 7.0 4.1 2.3 3.2 2.9 5.4 3.4 4.4 Table 4.2 Average carboxylic acid surface concentration on the surface of heated and UV and ozone treated polystyrene and PMMA. No Heat 90 95 Average 100 No Heat 90 95 Standard Deviation 100 Polystyrene PMMA 11.8 9.8 6.7 9.9 7.2 9.8 6.8 9.7 1.71 0.10 0.94 0.36 0.96 0.15 0.82 0.11 61 Table 4.3: Average Water Contact Angles for UV and Ozone Treated Polystyrene samples stored in various buffers at acidic, neutral, or basic pH. Average Standard Deviation 5 6 7 8 9 55.9 53.9 Water 55.2 57.1 59.1 61.7 PBS 57.2 60.0 62.4 HEPES Sodium 59.8 64.9 61.8 Borate 52.6 54.0 55.6 Tris 4.2 3.6 5.2 Water 2.8 3.8 4.7 PBS 4.9 5.71 5.9 HEPES Sodium 5.3 4.6 3.4 Borate 4.5 4.5 5.0 Tris Table 4.4: Average carboxylic acid surface concentration for UV and Ozone Treated Polystyrene samples stored in various buffers at acidic, neutral, or basic pH. Average Standard Deviation Water PBS HEPES Sodium Borate Tris Water PBS HEPES Sodium Borate Tris 5 13.5 10.7 6 7 15.4 7.2 6.1 5.0 8 9 9.0 7.1 5.9 6.0 5.1 6.1 6.6 7.8 6.3 3.26 0.48 1.65 0.55 1.43 1.28 1.49 0.26 0.52 0.49 0.97 1.32 0.43 0.20 1.33 62 REFERENCES 1. Zheng, W., Wong, S.C., and Sue, H.J. Transport Behavior of PMMA/Expanded Graphite Nanocomposites. Polymer, 73, 2002. pp. 6767-6773. 2. Piruska, A., Nikcevic, I., Lee, S.H., et. al. The Autofluorescence of Plastic Materials and Chips Measured Under Laser Irradiation. Lab on a Chip, 5, 2005. pp. 1348-1354. 3. Tsao, C.W., Hromada, L., Liu, J., et. al. Low Temperature Bonding of PMMA and COC Microfluidic Substrates Using UV-Ozone Surface Treatments. Lab on a Chip, 7, 2007. pp. 499-505. 4. Diaz-Quijada, G.A., Peytavi, R., Nantel, A., et. al. Surface Modifications of Thermoplastics- Towards the Plastic Biochip For High Throughput Screening Devices. Lab on a Chip, 7, 2007. pp. 856-862. 5. Sharp, J.S., Teichroeb, J.H., Forrest, J.A. The Properties of Free Polymer Surfaces and Their Influence on The Glass Transition Temperature of Thin Polystyrene Films. The European Physical Journal E, 15, 2004. pp. 473-487. 6. Toepke, M.W. and Beebe, B.J. PDMS Adsorption of Small Molecules and Consequences In Microfluidic Applications. Lab on A Chip, 6, 2006. pp. 14841486. 7. Mesfin, T., Taylor, P.L. Glass Transition Temperature for PMMA from Molecular Dynamics Simulations. NASA and APS Physics Meeting, March 2001. 8. Bliznyuk, V., Assender, H., Briggs, A., et. al. Surface Glass Transition Temperature of Amorphous Polystyrene Measured by SFM. APS Meeting, March 2001. 9. Rhodes, B.T. Burning Rate and Flame Heat Flux for PMMA in the Cone Calorimeter. Department of Commerce, 1994. 63 10. Bhattacharyya, A., Klapperich C.M. Mechanical and Chemical Analysis of Plasma and Ultraviolet-Ozone Surface Treatments for Thermal Bonding of Polymeric Microfluidic Devices. Lab on a Chip, 7, 2007. pp. 876-882. Curtis, A.S.G., Forrester, J.V., McInnes, C., et. al. Adhesion of Cells to Polystyrene Surfaces. J. Cell Biol., 97, 1983. 1500-1506. 11. Brown, H.R., Char., K., Deline, V.R., et. al. Effects of a Diblock Copolymer on Adhesion Between Immiscible Polymers. 1. PS-PMMA Copolymer Between PS and PMMA. Macromolecules, 26, 1993. pp. 4155-4163. 12. Wu, H. Zhang, R., Sun, Y., et. al. Biomimetic Nanofiber Patterns with Controlled Wettability. Soft Matter, 4, 2008. pp. 2429-2433. 13. Hwang, D.K., Moon, J.H., Shul, Y.G., et. al. Scratch Resistant and Transparent UV-Protective Coating on Polycarbonate. Journal of Sol-Gel Science and Technology, 26, 2003, 783-787. 14. Vestal, C.R. and Zhang, Z.J. Atom Transfer Radical Polymerization Synthesis and Magnetic Characterization of MnFe2O4/Polystyrene Core/Shell Nanoparticles. Journal of American Chemical Society, 124, 2002. pp. 1431214313. 15. Grohens, Y., Hammon, L, Carriere, P., et. al. Tacticity and Surface Chemistry Effects on The Glass Transition Temperature of Thin Supports PMMA Films. Mat. Res. Soc. Symp., 629, 2000. pp. 1.7.1-1.7.7. 16. Callen, B.W., Ridge, M.L. Lahooti, S. Remote Plasma and Ultraviolet-Ozone Modification of Polystyrene. J. Vac. Sci. Tehnol. A., 13, 4, 1995. pp. 20232029. 64 17. 2029. iii