Growth of Polystyrene Films via Gas-Phase Polymerization
advertisement
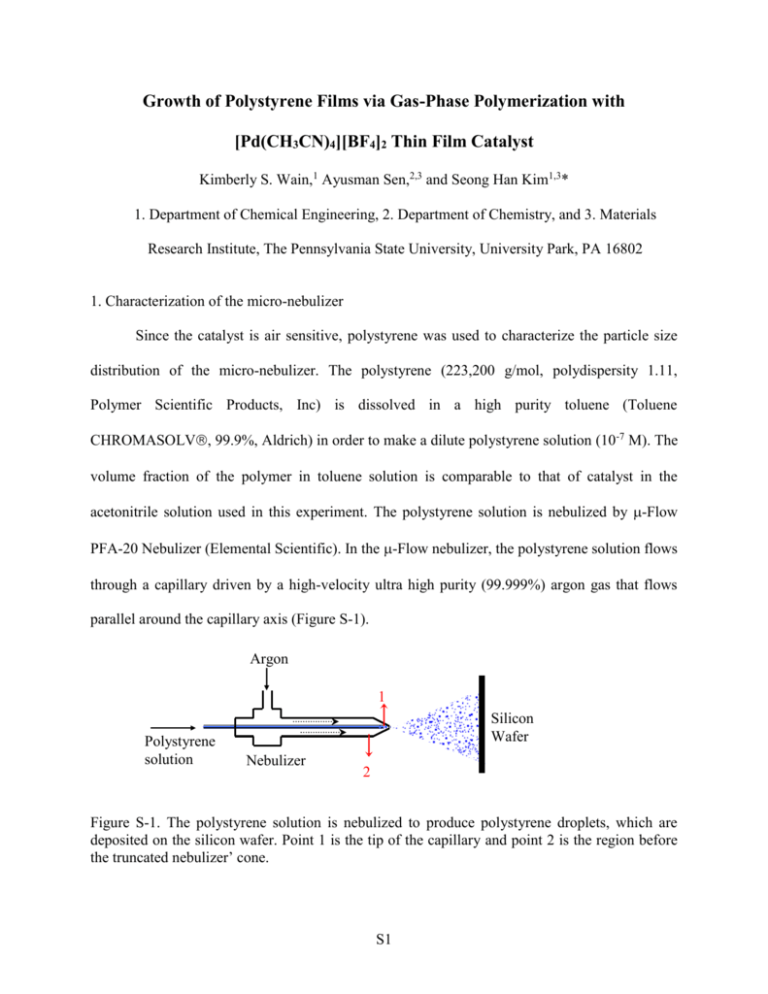
Growth of Polystyrene Films via Gas-Phase Polymerization with [Pd(CH3CN)4][BF4]2 Thin Film Catalyst Kimberly S. Wain,1 Ayusman Sen,2,3 and Seong Han Kim1,3* 1. Department of Chemical Engineering, 2. Department of Chemistry, and 3. Materials Research Institute, The Pennsylvania State University, University Park, PA 16802 1. Characterization of the micro-nebulizer Since the catalyst is air sensitive, polystyrene was used to characterize the particle size distribution of the micro-nebulizer. The polystyrene (223,200 g/mol, polydispersity 1.11, Polymer Scientific Products, Inc) is dissolved in a high purity toluene (Toluene CHROMASOLV, 99.9%, Aldrich) in order to make a dilute polystyrene solution (10-7 M). The volume fraction of the polymer in toluene solution is comparable to that of catalyst in the acetonitrile solution used in this experiment. The polystyrene solution is nebulized by -Flow PFA-20 Nebulizer (Elemental Scientific). In the -Flow nebulizer, the polystyrene solution flows through a capillary driven by a high-velocity ultra high purity (99.999%) argon gas that flows parallel around the capillary axis (Figure S-1). Argon 1 Polystyrene solution Silicon Wafer Nebulizer 2 Figure S-1. The polystyrene solution is nebulized to produce polystyrene droplets, which are deposited on the silicon wafer. Point 1 is the tip of the capillary and point 2 is the region before the truncated nebulizer’ cone. S1 The Scanning Mobility Particle Sizer (TSI Incorporated) measures the polystyrene nanoparticles’ size distribution. The size distribution shows a broad polystyrene nano-particle size distribution in all argon flow rates (Figure S-2). Furthermore, the increment in argon flow rate leads to less polystyrene nano-particles produced as a result of the decreasing polystyrene solution flow rate. The size distribution curved shifts to the smaller size as the argon flow rate increases, which indicates that the particle mean diameter decreases as the ratio of liquid to gas flow rate increases (80, 78, 70 and 70 nm for argon flow rate 1.30, 1.75, 2.26 and 2.80 lt/min respectively). #/cm3 Size distribution of 1*10^-7 M PS aerosols 2.50E+06 2.00E+06 1.50E+06 1.00E+06 5.00E+05 0.00E+00 20 psi 30 psi 45 psi 60 psi 0 100 200 300 nm Figure S-2. The size distribution of polystyrene nano-particles (aerosol) at argon pressure gauges of 20, 30, 45 and 60 psig, which correspond to argon flow rates of 1.30, 1.75, 2.26 and 2.80 lt/min respectively. The x axis is the particle’s diameter, while the y axis is the number of particle in a unit volume of solvent [45]. S2 2. SEM images of the [Pd(CH3CN)4][BF4]2 catalyst film The catalyst film was deposited with the -Flow nebulizer using 10-3M acetonitrile solution and coated with 10~20nm thick gold for SEM imaging (Figure S-3). The catalyst film is decomposed and delaminated under the high magnification electron beam irradiation. (b) (a) Figure S-3. (a) SEM image of the pristine catalyst film imaged at a magnification of x2500. (b) SEM image of the same area at the same magnification after attempt to obtain a higher resolution at x4000. The center region was exposed to the x4000 electron beam for 30 seconds. S3










