Practical Aspects of Light Absorption
advertisement
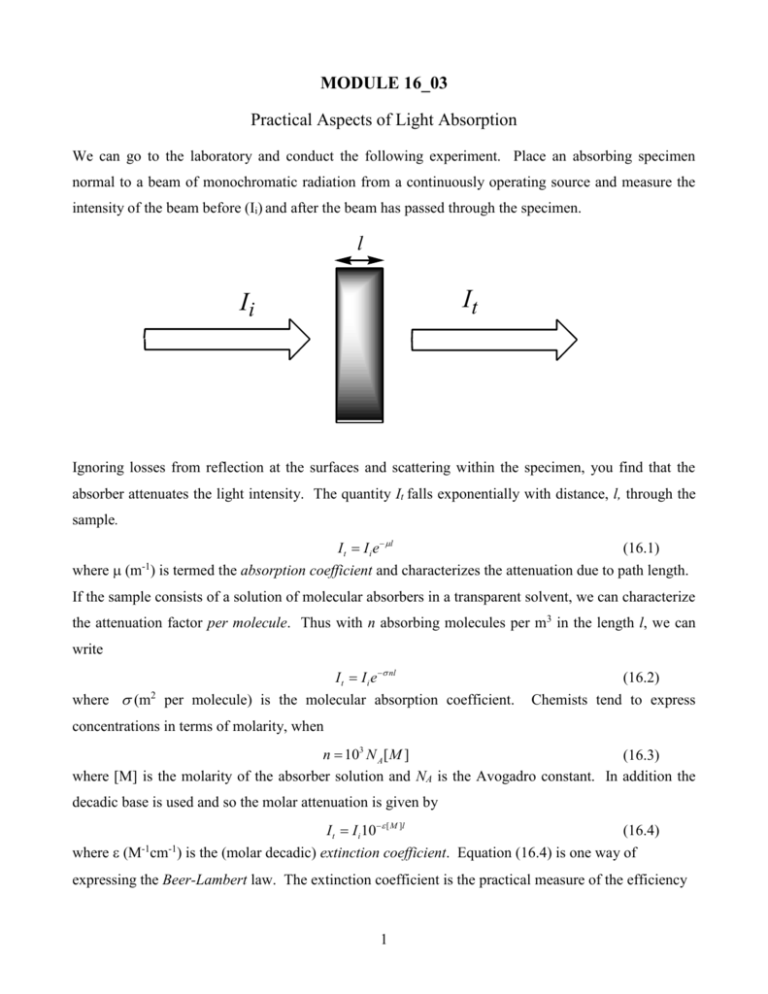
MODULE 16_03 Practical Aspects of Light Absorption We can go to the laboratory and conduct the following experiment. Place an absorbing specimen normal to a beam of monochromatic radiation from a continuously operating source and measure the intensity of the beam before (Ii) and after the beam has passed through the specimen. l It Ii Ignoring losses from reflection at the surfaces and scattering within the specimen, you find that the absorber attenuates the light intensity. The quantity It falls exponentially with distance, l, through the sample. I t I i e l (16.1) where (m ) is termed the absorption coefficient and characterizes the attenuation due to path length. -1 If the sample consists of a solution of molecular absorbers in a transparent solvent, we can characterize the attenuation factor per molecule. Thus with n absorbing molecules per m3 in the length l, we can write I t I i e nl where m per molecule) is the molecular absorption coefficient. 2 (16.2) Chemists tend to express concentrations in terms of molarity, when n 103 N A [ M ] (16.3) where [M] is the molarity of the absorber solution and NA is the Avogadro constant. In addition the decadic base is used and so the molar attenuation is given by I t I i 10 [ M ]l (16.4) where (M cm ) is the (molar decadic) extinction coefficient. Equation (16.4) is one way of -1 -1 expressing the Beer-Lambert law. The extinction coefficient is the practical measure of the efficiency 1 with which a molecule, or a chromophoric entity, absorbs light. It varies from chromophore to chromophore, and is a function of the wavelength of the light that is applied. Another way of expressing equation (16.4) is in logarithmic form I log i [ M ]l A It where A is termed the absorbance of the absorbing entity (sample). (16.5) Figure 16.1 shows plots of the absorbance as a function of wavelength for solutions of benzene and anthracene in hexane solvent. An absorption spectrophotometer was employed to obtain the plots. In the experiment the concentration and the path length were fixed so the amplitude variations arises from variation of the extinction coefficient with wavelength. The reason for this variation will be examined later. 1.0 0.6 anthracene benzene 0.5 0.8 absorbance absorbance 0.4 0.3 0.6 0.4 0.2 0.2 0.1 0.0 0.0 220 225 230 235 240 245 250 255 260 265 270 275 280 280 300 320 340 360 380 400 420 440 wavelength/nm wavelength/nm FIGURE 16.1 In many cases we, as photoscientists are interested in the quantity of light that is absorbed by a sample. Ignoring scattering and reflections we can see that Ii It I a (16.6) where Ia is the light absorbed in the sample during the exposure. Combining equation (16.4) with (16.6) we obtain I a Ii It I i (1 10 [ M ]l ) I i (1 10 A ) (16.7) The quantities Ia and It can be expressed either as the total number of photons accumulated over the exposure time (note that NA of photons = 1 einstein), or as a rate, in photons per second. 2 Since the absorption of one photon by a molecule generates one primary excited state, then the rate of primary excited state production equals the rate of photon absorption. d [ M *] I a I i (1 10 A ) dt (16.8) in units of (molecules) per second. The connection between theory and experiment At this point it is useful to relate the experimental quantity to the theoretical quantities B( B f i B f i ) and fi. Consider a plane of area A at a position x with radiation incident on the plane from the left. All photons within a length ct, (c = speed of light) and hence in a volume Act will pass through the plane in the interval t. If the energy density of the field is U, then the total electromagnetic energy passing through the plane in t is Uact. The energy flux, F, is the energy per unit time per unit area and so F UAct / At cU (16.9) In the frequency range to +d the energy density is dU rad ( E )d (16.10) and so the energy flux in the same range is dF c rad ( E )d . If we write dF I ( )d where I is the radiation intensity, then I c rad . Now let us consider the absorption that occurs within a slab of thickness dl. Let the total number of molecules in the slab that can absorb radiation between and d be n(dso the total number density of absorbers is N n( )d . The rate at which a given molecule absorbs a photon is ku l B rad ( Eul ) . Each photon has energy h, and so the rate of change of energy density is dU h k f i n( )d n( )h B rad ( E )d dt The energy entering the slab at x from the left in dt is F(x)Adt, and the energy leaving the slab on the right at x+dl is F(x+dl)Adt. The difference between these two quantities is the rate of change of energy in the slab volume and is given by Rearranging we get d (UAdl ) F ( x dl ) A F ( x) A dt (16.12) dU F ( x dl ) F ( x) dF dt dl dl (16.13) 3 dF dI d . By using equation (16.7) we can obtain dl dl n( )h dI n( )h B rad dl BIdl (16.14) c We also are aware that the reduction in light intensity by a beam on passing through a solution of Remembering that I c rad , and therefore length dl containing absorbing molecules of concentration [M] is dI ( )[ M ]Idl (16.15) ( ) hn( ) B c[ M ] (16.16) see Integrating both sides with respect to over all the frequencies in the radiation field leads to ( ) BhN BhN (16.17) cA c A For typical absorption bands () is non-zero over a very narrow range of frequencies, so it is not too much of an approximation to set = fi. On doing this and putting J ( ) d we have J h fi / c N A B (16.18) In an earlier module we showed that the Einstein B coefficient is related to the transition dipole moment according to 2 B fi / 6 0 2 thus J fi N A fi 2 (16.19) 3 0 c Thus we have established a connection between the integrated extinction coefficient (a measurable quantity) and the theoretical quantities B and the transition dipole moment. The final thing to do is to introduce the oscillator strength of a transition, f, which is a dimensionless quantity. 4 me fi f 2 3e and combining this with equation (16.19) we obtain 4m c f e 20 J N Ae fi 2 6.257x1019 x( J / m 2 mol 1s 1 ) In practice, for allowed electric dipole transitions, f = 1; for forbidden transitions f<<1 4 (16.20) (16.21)