Topical therapy prevents skin rejection in composite tissue
advertisement

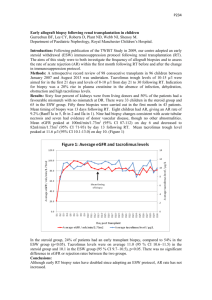
Overcoming Skin Immunogenicity in Transplantation Tolerance Mario G. Solari MD, Kia M. McLean MD, Justin M. Sacks MD, Theresa Hautz, Jignesh V. Unadkat MD, Elaine K. Horibe MD, Angus W. Thomson PhD, DSc, W.P. Andrew Lee MD Introduction: Skin is the most immunogenic component of a composite tissue allograft (CTA). Clinically this has manifested as multiple acute skin rejection episodes in most of the human CTA performed to date. Intravenous steroids and increased systemic immunosuppression have been used to mitigate these rejection episodes. These drugs cause direct organ toxicity and are associated with metabolic dysfunction, opportunistic infection, and malignancy. Topical immunotherapy is an attractive and practical therapeutic option to provide local immunosuppression with minimal systemic toxicity. Topical tacrolimus is currently FDA approved to treat atopic dermatitis, which is indistinguishable from acute rejection histologically. Atopic dermatitis is characterized by a high proportion of antigen presenting cells (APC) that exhibit high stimulatory activity toward autologous T cells. This activity is strongly reduced after the application of topical tacrolimus, as is the number of infiltrating immune cells and overall inflammatory cytokine production. The present study applies these properties of topical tacrolimus to CTA. It investigates the potential of topical tacrolimus to maintain a CTA after total withdrawal of a short course of systemic therapy. Methods: Wistar Furth to Lewis (full MHC mismatch) orthotopic hind limb transplants were performed. Groups included: I- topical tacrolimus alone, II- anti-lymphocyte serum (ALS) (0.5mL x2 doses) + 21 days cyclosporine (CsA) (10/mg/kg/day), III- ALS (2 doses) + 21 days CsA + topical tacrolimus once daily. The endpoint of the study is grade 3 rejection, defined by epidermolysis, or 100 days (long term survival). Biopsies (Fig 1&2) of skin, muscle, and bone were taken for immunohistochemistry and H&E. Results: All animals in Group I (n=7) developed grade 3 clinical rejection by postoperative day (POD) 9, similar to controls without treatment. The mean onset of grade 3 rejection was POD 40 with a range of 34-44 in Group II (n=7). In Group III (n=6), two animals developed grade 3 rejection on POD 35 and 56. The remaining 4 experimental animals reached the 100 day endpoint without grade 3 rejection (Fig 3). Conclusion: This study demonstrates the feasibility of maintaining a CTA on topical tacrolimus therapy alone after induction therapy. The induction protocol in this model mirrors what is currently performed clinically where recipients undergo lymphoid depletion before organ transplantation, followed by systemic immunosuppression. Preoperative depletion of T cells with ALS, along with a short course of systemic immunosuppression, prevents acute rejection, while topical tacrolimus may inhibit immune cell activation and multiplication in the skin component of the CTA. This novel regimen could reduce or eliminate the morbidity associated with systemic immunosuppression in clinical CTA. Figure 1. Representative histology from a group 1 animal at grade 3 rejection. a) The muscle is infiltrated with lymphocytes with perivascular concentration. b) The skin is heavily infiltrated with lymphocytes and the epidermis absent secondary to epidermolysis. Figure 2. A representative long-term survivor from group 3 at POD 102. a) The transplanted limb is in the foreground and near indistinguishable from the native limb in the background. b) muscle and c) skin are grossly normal with scattered lymphocytes. The epidermis is intact with a healthy basal keratinocyte layer. Figure3. Limb allograft survival data from all three groups. References: 1. Wollenberg A, Sharma S, von Bubnoff D, Geiger E, Haberstok J, Bieber T. Topical tacrolimus (FK506) leads to profound phenotypic and functional alterations of epidermal antigen-presenting dendritic cells in atopic dermatitis. J Allergy Clin Immunol. 2001 Mar;107(3):519-25. 2. Simon D, Vassina E, Yousefi S, Kozlowski E, Braathen LR, Simon HU. Reduced dermal infiltration of cytokine-expressing inflammatory cells in atopic dermatitis after short-term topical tacrolimus treatment. J Allergy Clin Immunol. 2004 Oct;114(4):887-95. 3. Fujita T, Takahashi S, Yagihashi A, Jimbow K, Sato N. Prolonged survival of rat skin allograft by treatment with FK506 ointment. Transplantation. 1997 Sep 27;64(6):922-5.