organic sample test
advertisement

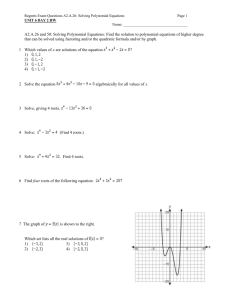
Organic Chemistry Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. ____ ____ 1. What results when a secondary alcohol is oxidized? a. a ketone d. an acid b. an amine e. no reaction c. an aldehyde 2. Which type of reaction will an alkene not undergo? a. addition d. dehydration b. polymerization e. hydration c. oxidation/reduction 3. Which of the following classes of organic compounds does not contain oxygen? a. aldehydes d. ethers b. amines e. amides c. amino acids 4. The correct IUPAC name for the compound below is which of the following? ____ a. 1,1-diiodo-2-fluoro-3-cycloproplycyclobutane b. 1-cyclopropyl-2-fluoro-3,3-diiodocyclobutane c. 1,1-diiodo-3-cyclopropyl-4-fluorocyclobutane d. 1-fluoro-2,2-diiodo-4-cyclopropylcyclobutane 5. The synthesis sequence shown here is best described as which of the following? ____ ____ 1. ____ ____ ____ 2. 3. a. (1) Dehydration; (2) halogenation; (3) hydrogenation b. (1) Hydrogenation; (2) dehydration; (3) halogenation c. (1) Hydrogenation; (2) halogenation; (3) dehydration d. (1) Halogenation; (2) hydrogenation; (3) dehydration e. (1) Dehydration; (2) hydrogenation; (3) halogenation 6. Ethanoic acid (vinegar) when diluted to low concentrations by water can be prepared from ethene by a. reduction with H2, followed by reaction with a strong oxidizer b. addition of HCl, followed by reaction with H2O c. addition of H2O followed by reaction with a strong oxidizer d. addition of Br2, followed by reduction with H2 7. How many moles of carbon dioxide would result from the complete combustion of one mole of fructose (C6H12O6)? a. 0 c. 6 b. 5 d. 7 8. What is the monomer for the polymer Kel-F shown below? ____ a. c. b. d. 9. Which of the following polymers is a polyester with 4 carbons in the dialcohol and 3 carbons in the dicarboxylic acid? a. b. c. d. ____ 10. When CH4 is reacted with excess Cl2(g) in the presence of light, the reaction mixture would most likely contain which of the following? a. CH3Cl c. CH3Cl, CH2Cl2, CHCl3 b. CH2Cl2 d. CHCl3 Completion Complete each sentence or statement. 11. The correct IUPAC name for the structure: is ______________________________. 12. The primary reaction product of the reaction given below is (draw structure)____________. 13. When (CH3)3COH is added to an oxidizing agent, the functional group found in the reaction vessel afterward is ____________________. 14. The functional group formed by amino acids in a protein is ____________________. 15. The presence of OH and NH groups in an organic compound leads to _________________________ between molecules. Short Answer 16. Communication - (5 marks) Make a summary table of five organic functional groups using the heading: Name, Functional Group (structure), IUPAC Suffix/Prefix. (5 marks) 17. Application - (11 marks) PABA, properly known as 4-amino-1-benzoic acid, is found in sunscreen lotions. Draw the condensed formula for this compound. What allows it to bind to the skin and the lotion's other components? (3 marks) 18. Years in the future, you venture into a long forgotten landfill to find antique bottles. As you dig, you unearth a plastic toy (polyethylene) you threw away when you were six years old. Why would this toy still be largely intact? (2 marks) Essay 19. The pulp and paper industry is a major employer in many small towns in Northern Ontario and other provinces. Some of the by-products of the pulp process include short chain carboxylic acids such as propanoic and butanoic acid. These are produced in quantities large enough that the strong odour produced by these acids in local streams from waste water causes concern among town residents. As an organic chemist, devise two strategies to deal with this problem and show appropriate reactions. Both strategies should include 1) containment and 2) treatment. (6 marks) Case (13 marks) 20. Give the condensed formula and the IUPAC name for: (2 marks each) a) CH3- COOH + CH3CH2-NH2 → b) CH3-CHO + H2, Pt → c) CH3-CH2-CH3 + Cl2, heat → d) CH3 – CH2 – CH – OH + [O] → | CH3 e) O CH3 || | CH3-CH2-CH2-C-CH-CH3 + [O] → f) Balance the following reaction (4 marks) 3 methyl-2 butanol is oxidized in acid to a ketone using Cr2O72- (Cr3+ is the product). Organic Chemistry Answer Section MULTIPLE CHOICE 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: A D B A E C C C A C REF: REF: REF: REF: REF: REF: REF: REF: REF: REF: K/U K/U K/U C K/U C C C C C OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: OBJ: 1.6 1.3 1.1 1.4 1.9 1.9 2.5 2.1 2.2 1.4 COMPLETION 11. ANS: 3,3,6-trimethylnonane REF: C OBJ: 1.2 LOC: OC2.02 REF: C 13. ANS: (CH3)3COH OBJ: 1.3 LOC: OC2.05 REF: C 14. ANS: amide OBJ: 1.5 LOC: OC2.05 REF: K/U OBJ: 1.8 15. ANS: hydrogen bonding LOC: OC1.01 12. ANS: REF: K/U OBJ: 1.1 LOC: OC1.02 SHORT ANSWER 16. ANS: Name alcohol ether Functional Group IUPAC Suffix -ol -oxy LOC: LOC: LOC: LOC: LOC: LOC: LOC: LOC: LOC: LOC: OC1.03 OC1.03 OC1.01 OC2.02 OC1.03 OC2.05 OC2.05 OC2.05 OC2.05 OC2.05 aldehyde -al ketone -one carboxylic acid -oic acid ester -oate amine -amino amide -amide REF: C 17. ANS: - OBJ: 1.1 LOC: OC2.01 hydrogen bonding by the amine group and the OH group in the carboxylic acid allow it hydrogen bond with the skin REF: MC OBJ: 1.8 LOC: OC3.03 18. ANS: - plastics resist biodegradation - they are resistant to heat, chemical reactions and bacteria - most plastics will take generations to break down into simpler products - this reinforces the need for recycling REF: MC OBJ: 2.1 LOC: OC3.03 ESSAY 19. ANS: - do not dump the 'waste' water directly into the streams but place in holding lagoons - in this way, the water can be treated before being released into streams - carboxylic acids can be reduced into biodegradable alcohols carboxylic acids can be reacted with alcohols selected to make esters which do not have foul odours and are inviting to bacteria which will consume them react the carboxylic acids with a base to make an insoluble salt which can be removed from the bottom of the lagoon, and the clear water on top can be released into the stream REF: MC 20. OBJ: 1.7 a) CH3-CO-NH-CH2-CH3 b) CH3CH2OH c) eg. LOC: OC3.03 N-ethylethanamide ethanol CH3-CH-CH3 | Cl d) CH3-CH2-CO-CH3 e) N.R. f) 3-8-1→ 2-3-7 2-butanone