CL-clown
advertisement

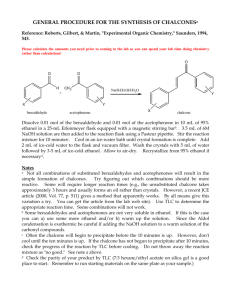
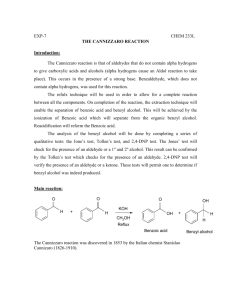
This journal is © The Royal Society of Chemistry 2000 1 Synthesis & Ionic Properties of Nematic Compounds Bearing an Ether-Crown Moiety : an NMR Approach Supplementary Materials Section : The synthetic scheme of these three series is presented in the following Schemes HO C 6H 13O OH OC 6H 13 H a) H C 6H 13O C 6H 13O O O OH 13C 6 H b) C 6H 13O HO CH 3 NO 2 NO 2 H c) d) HO O 3 OH O Cl 3 O O e) Cl O C 6H 13O H O O HO + OH 13C 6 H C 6H 13O NH 2 O H H HO O A B f) O O O O O H O O O N Scheme 1 : Synthetic scheme for CBS6 compound a) C6H13Br, NaOH, H2O, phase transfer catalyst ; b) tBuOK, polyethylene glycol 200 ; c) Zn, EtOH/H20, CaCl2 ; d) SOCl2/DMF ; e) NaOH, EtOH ; f) EtOH. C 6H13O H a) NH 2 C 6H13O O O b) H O O R OH H O Na R A O R N N H OC 6H 13 C 6H13O O OC 6H13 H C 6H 13O O O O O c) OH O R = H, CH 3, OCH 3, OC 6H13 R O O O O Cl d) O O O B O O O O O O O N O O O N R R Scheme 2 : Synthetic scheme for CRA6 compounds. a) NaNO2 , HCl, dioxane; b) KMnO4, H2O i) NaOH ii) HCl ; c) SOCl2 ; d) CHCl3, pyridine. 2,3,4-Trialkoxybenzaldehyde was prepared in one step by total etherification of 2,3,4trihydroxybenzaldehyde using the phase transfer catalysis method. The reaction was carried out in water under nitrogen by mixing the aldehyde, 3.5 eq. of bromohexane and Aliquat 336 as phase transfer catalyst. The solution was brought to 80°C and a solution of 3.5 eq. of NaOH in water was added over a 4 hours period. The reaction time was 12 hours. After chloroform extraction, the aldehyde was chromatographed This journal is © The Royal Society of Chemistry 2000 2 on silica gel (60-200 mesh) with CH2Cl2 as eluent. The 2,3,4 trialkoxybenzaldehyde (1 eq. mol) and 4nitrotoluene (1.5 eq) were dissolved in PEG 200 at 90°. An homogeneous solution of potassium tbutoxide (1.5 eq. mol) in PEG was then slowly added to the above solution. The reaction mixture was left at 90°C for 48 hours. After cooling, the PEG phase was extracted with CH2Cl2 and washed three times with acidified water. Pure 2,3,4-alkyloxy-4'-nitrostilbene was obtained after chromatography on silica gel with CH2Cl2/heptane (50/50) as eluent. 2,3,4-Alkyloxy-4'-aminostilbene (B) was then obtained by reduction of the nitro group using zinc in ethanol under neutral conditions. The 4'-formylbenzo-15-crown-5 (A) was synthesized following the procedure described by Pedersen. Tetraethyleneglycol was allowed to react with a large excess of thionyl chloride and few drops of DMF at reflux during 6 hours. Then, excess of thionyl chloride was removed under vacuum and pure 1,11dichloro-3,6,9 trioxaundecane was obtained after washing several times with a KHCO3 solution. A mixture of 3,4-dihydroxybenzaldehyde and 3 eq. of NaOH in EtOH was stirred and heated to reflux under nitrogen. Then an ethanolic solution of the dichloro compound was slowly added and the mixture was heated under reflux for 48 hours. Ethanol was evaporated, the residue was dissolved in CH2Cl2 and washed with basic solution. Crude (A) is obtained and purified by chromatography on silica gel with CH2Cl2/ethyle acetate (50/50) mixture. The corresponding acid 4'-carboxybenzo-15-crown-5 was obtained by oxidation with KMnO4. The carboxylic acid was purified by crystallization in water and acetone. For the CBSn, the Schiff base was obtained by reacting the 2,3,4-alkyloxy-4'-aminostilbene with 4'formylbenzo-15-crown-5 (A) in boiling ethanol. The Schiff base was obtained by reacting 2,3,4trialkoxybenzaldehyde with the corresponding diamine in boiling ethanol. The final product was recrystallized in ethanol until constant transition points were obtained. Coupling of the diazonium salt to the phenate was effectued in dioxane. The compound was extracted in CH2Cl2 and chromatographed on silica gel (60-200 mesh) with CH2Cl2/ethylacetate (50%) as eluent. In the next step one equivalent of the acetyl chloride derivative of (A) was added to the modified stilbene in CHCl3/pyridine to give the CRA6. Then the pure liquid crystalline product was chromatographed on silica gel (60-200 mesh) with CH2Cl2/ethylacetate (50%) and recrystallized in a mixture of chloroform and ethanol until constant transition points were obtained. All the compounds were characterized by 1H, 13C and mass spectrometry using the electrospray ionization method.