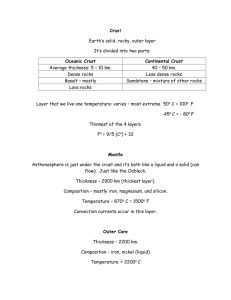
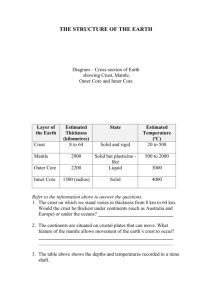
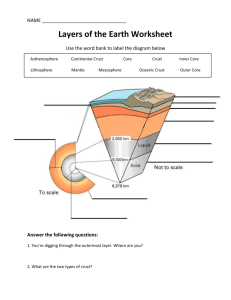
Acknowledgement: The project is supported by fund J04/98
advertisement

THERMAL PRESSURE FORMING OF STARCH BASED PACKAGING MATERIALS Zitný R.1, Šesták J.1, Tsiapouris A.2, Linke L.2 1 CTU , Faculty of mechanical engineering, Technická 4, 166 07 Prague 6 2 Dresden University of Technology, Faculty of mechanical engineering, Institute of Food Engineering and Bio-Engineering, D-01062 Dresden E-mail: zitny@fsid.cvut.cz Phone: (+42 02) 2435 2547 Fax: (+42 02) 2431 0292 E-mail: sestak@fsid.cvut.cz Phone: (+42 02) 2435 2547 Fax: (+42 02) 2431 0292 E-mail: Tsiapouris@mvll00.mw.tu-dresden.de Phone: (+49 0351) 463 6158 Fax: (+49 0351) 463 7126 E-mail: Linke@mvll00.mw.tu-dresden.de Phone: (+49 0351) 463 4985 Fax: (+49 0351) 463 7126 Scientific topic: II.4. Abstract: Thermal pressure forming of starch based water suspension was investigated experimentally and theoretically. Processed material (thin plates) was baked in a metallic mould having a constant temperature of walls. Time courses of temperature and pressure inside the samples were recorded and compared with the suggested crust and core model. Keywords: starch, packages, waffle-baking, crust-core model, biodegradation INTRODUCTION Thermal pressure forming of the starch based biologically degradable products (e.g. trays, plates, packages, etc.) is similar to the contact baking of waffles in a toaster: Raw material - water suspension of starch and additives (e.g. cellulose fibres) - is placed into a closed planar mould and heated by contact with walls having a constant temperature (180-1900C). The phenomena taking place inside the heated layer are rather complicated, and the following three phases can be distinguished: Heating of liquid suspension up to the boiling point. Evaporation of free water accompanied by significant increase of pressure inside the mould. Expansion of material (initial volume of suspension is increased 2-3 times). Crust formation. Remaining bound water removal. Pressure decreases, temperature increases. Process control, process design and optimisation require assessment of the influence of product dimensions (thickness of material) and wall temperature on the process time and the quality of product. EXPERIMENTS 1 Experimental set-up An experimental heater set-up is T[ C] p[bar] shown in Fig.1. The cavity in the mould is a rectangular box 270 x 200 x 2, 4 and 8 mm, having venting channels around its t[s] t[s] periphery. Both the lower and the upper pC - crust metallic plates are heated electrically and their temperature is monitored by thermopG - gap couples. The temperature of plates is maintained at approximately 180-1900C. The crust second pair of thermocouples is located inside the cavity, the first thermocouple in 4 mm the centre (approximately 130 mm from the shorter side) and the second in the vicinity of the shorter side. This second thermocouple 130 mm enables to estimate the time, when the front Temperature at interface is Liquid core flows of the expanding sample arrives to the side controlled by pC+pG driven by pressure wall of mould. Pressure of steam is detected D=150270 mm by a pressure transducer, which is flash mounted at the centre of the upper plate. Fig.1 Baking in a mould - geometry 2 Measurement procedure - results Tested samples, water suspensions of potato starch and additives, were inserted into the centre of the mould (mass of samples varied from 80 to 120 g, usually 114 g, for 4 mm thickness). After closing the mould the sample forms a circular disk of diameter approximately 160 mm. It was found experimentally that this amount of suspension is sufficient to fill the whole cavity at the end of baking. Corresponding volumetric expansion is approximately 2.7 times. 0 Initial composition of samples varied, but the concentration of solid phase was always the same, 40% (i.e. 60% water w/w). Though a lot more additives were tested (e.g. xanthan, carboxy-methyl-cellulose, methylstearat, different cellulose fibres, waxes etc.) only the results for pure potato starch suspension (denoted KS) and a mixture of 30% potato starch and 10% cellulose fibres (denoted PS) are presented in Figs.2a, 2b, 2c, 2d. Time courses of temperatures (at the side of mould and in the centre) and pressures were evaluated as time averages from several repeated measurements. Centre PS-2 Paper Fibres: 10% - Thickness: 2 mm Pressure Centre Pressure 3,0 20 2,0 20 2,0 0 1,0 0 2:30 2:24 2:18 2:12 2:06 2:00 1:54 1:48 1:42 1:36 1:30 1:24 1:18 Fig.2b Starch 30%, paper 10%, thickness 2 mm Starch 40%, thickness 2 mm KS-4 Potato Starch: 40% - Thickness: 4 mm 1:12 Time [min:s] Vicinity Centre Paper Fibres: 10% - Thickness: 4 mm Pressure PS-4 Vicinity Centre Pressure Fig.2c 5:00 4:48 4:36 4:24 4:12 4:00 3:48 3:36 3:24 3:12 3:00 2:48 2:36 2:24 2:12 2:00 1:48 1:36 0:00 5:00 Time [min:s] 1:24 1,0 1:12 0 1:00 1,0 0:48 2,0 0 0:36 20 0:24 2,0 0:12 3,0 20 Temperature [°C] 40 Pressure [bar] 3,0 4:48 4,0 40 4:36 60 4:24 4,0 4:12 5,0 60 4:00 80 3:48 5,0 3:36 6,0 80 3:24 100 3:12 6,0 3:00 7,0 100 2:48 120 2:36 7,0 2:24 8,0 120 2:12 140 2:00 8,0 1:48 9,0 140 1:36 160 1:24 9,0 1:12 10,0 160 1:00 180 0:48 10,0 0:36 11,0 180 0:24 200 0:12 11,0 0:00 200 Pressure [bar] Fig.2a 1:06 1:00 0:54 0:48 0:42 0:36 0:30 0:24 0:18 0:12 0:06 1,0 0:00 2:30 Pressure [bar] Temperature [°C] 40 2:24 3,0 2:18 4,0 40 2:12 60 2:06 4,0 2:00 5,0 60 1:54 80 1:48 5,0 1:42 6,0 80 1:36 100 1:30 6,0 1:24 7,0 100 1:18 120 1:12 7,0 1:06 8,0 120 1:00 140 0:54 8,0 0:48 9,0 140 0:42 160 0:36 9,0 0:30 10,0 160 0:24 180 0:18 10,0 0:12 11,0 180 0:06 200 Time [min:s] Temperature [°C] Vicinity 11,0 0:00 Temperature [°C] Vicinity Pressure [bar] KS-2 Potato Starch: 40% - Thickness: 2 mm 200 Time [min:s] Starch 40%, thickness 4 mm Fig.2d Starch 30%, paper 10%, thickness 4 mm Different behaviour exhibit relatively thick samples (8 mm) of pure potato starch (Fig.3). Centre Pressure Time [min:s] Fig.3 Starch 40%, thickness 8 mm 11:42 11:15 10:48 10:21 9:54 9:27 9:00 8:33 8:06 7:39 7:12 6:45 6:18 5:51 5:24 4:57 1,0 4:30 2,0 0 4:03 3,0 20 3:36 4,0 40 3:09 5,0 60 2:42 6,0 80 2:15 7,0 100 1:48 8,0 120 1:21 9,0 140 0:54 10,0 160 0:27 11,0 180 0:00 Temperature [°C] Vicinity Pressure [bar] KS-8 Potato Starch: 40% - Thickness: 8 mm 200 Some characteristic values are summarised in Tab.1 - process time, maximum pressure, temperature of suspension corresponding to the second phase of free water evaporation and also the mean velocity u of the edge during expansion of circular samples. This velocity u was estimated from the time delay of the side-wall thermocouple response. Tab.1 Experiments KS (native potato starch 40%), PS (starch 30%, paper fibres 10%), BEM (starch 27%, cellulose fibres type Arbocel BEM 10%, pre-gelatinised starch 3%) Material - Process time Boiling begins pmax p“ T u Thickness at time [mm] [s] [s] [bar] [bar] [0C] [mm/s] KS - 2 140 6 7.7 5.4 155 5.500 KS - 4 350 20 6.7 4.8 150 0.724 KS - 8 800 40 5.5 3.6 140 0.367 PS - 2 140 6 6.8 4.8 150 5.500 PS - 4 400 8 6.3 7.9 120-170 1.375 PS - 8 1000 1.8 1.4 110 0.519 BEM - 2 140 8 6.8 5.4 155 5.500 BEM - 4 300 10 3.9 3.6 140 1.964 BEM - 8 1000 1.8 1.3 107 1.727 The fifths column, p“, is calculated as the tension of saturated water vapour at temperature T in the sixth column of the table. It is rather difficult to measure temperatures inside the flowing core of sample (precise adjustment of thermocouples position) and even the interpretation of the pressure transducer reading has been subject of discussion. However, some conclusions seem to be solid: The temperature varies between 140 0C and 160 0C and is usually almost constant in the second phase of free water evaporation. The most surprising is the observation, that the temperature of a heated sample sometimes decreases even if the temperature of wall is substantially higher and almost constant. Besides the local maximum of temperature one or even two peaks of pressure were recorded. There exists some relationship between wall pressure and temperature of the flowing core during the second phase of baking, and the temperature time courses are controlled by the pressure drop of water steam. The expansion of heated materials is never accomplished during the first phase of heating and it seems to be probable that the expansion - flow of liquid core - is caused by bubbles formed in the suspension in the second phase of baking (boiling). MATHEMATICAL MODELLING 1 Review of mathematical models Mathematical models describing heat and mass transfer during frying or roasting of meat, and baking biscuit were presented by Farkas [1], Õzilgen [2], Singh [3],[4] or Zanoni [5]. There are some common features in these models, usually the assumption of two regions - dried crust and moisten core - with a moving interface between them. However, all the mentioned solutions are able to predict only monotone rising temperature profiles. The reason is that the overall pressure was assumed to be atmospheric, and no significant volumetric expansion of samples was expected. 2 Crust and core integral model Suggested crust and core model tries to explain some non-usual phenomena assuming that the boiling temperature of free water -and thus the temperature of suspension- is controlled by the pressure of the evaporated steam. The simplest model is integral, supposing spatially uniform temperature and moisture. This assumption seems to exclude the effects caused by the moving evaporation front between the crust and core, but this phenomenon can be included into the flow resistance and heat transfer resistance. This approach is probably the most significant contribution to the modelling of the baking process, as it removes severe numerical problems (instabilities) related to a strong non-linear coupling between the temperature and the pressure fields. The integral model accounts for the influence of finite heat capacity of the heating plates and related changes of the wall temperature, see Fig.4. Control volume: cylinder D0, height H hG(t) H Tw(t) h(t) crust core T(t) Mb(t) p(t) Mw(t) (t ) M v c (t ) M D0 D(t) Fig.4 Dmax Cylindrical sample Three phases of thermal processing are analysed separately: The first phase is heating of a more or less homogenous layer of water suspension up to the onset of boiling. The second phase is characterised by evaporation of free water and by formation of a growing solid crust. At the same time the liquid core expands, driven by pressure of steam, which leaves the crust-core interface and flows through the porous crust and along the walls of the mould. The friction loses in the crust and in the interstitial spaces at the wall determine pressure at the crust-core interface, and are manifested as the first peak of recorded pressure. The expansion rate of material is determined by the thickness of the liquid core, by viscosity of suspension and by the pressure at the crust-core interface. When the crust is fully developed and the expansion stops, the last phase of heating, accompanied by the evaporation of the remaining bound water, begins. The sample temperature no longer depends on the boiling temperature and steadily approaches the temperature of wall. 2.a Basic assumptions and equations Integral heat and mass balances were formulated for a constant control volume, confined by the symmetry plane and the surface of cylindrical sample, see Fig.4. The model assumes that: There is a thin gap of the width hG between the surface of cylindrical sample and the wall of mould. This gap filled by steam represents thermal and hydraulic flow resistance. Crust contains only solid, bound water and steam. Thermal conductivity of this porous layer depends on porosity c. Thickness of crust, h, can be calculated from material balance if the mass flow-rates of suspension, and the escaping steam are known. It is assumed that at the surface a very thin layer of melted starch is gradually built; this layer has negligible effect on heat transfer resistance, but influences pressure drop across the crust. Liquid core has a uniform temperature which is the same as the crust-core interface temperature. Suspension is a Newtonian liquid, which flows in the radial direction driven by internal pressure in bubbles. This pressure equals saturated vapour pressure at a temperature of core. Relative composition of solid, bound and free water content is independent of time, but the porosity of core c is changing. The most important and sufficiently general equation for the temperature time course T(t) follows from the enthalpy balance of the control volume shown in Fig.4, dH v (rw cw Tw ) ( M ss cs M bs cb M ws cw )T , e S (Tw T ) M (1) dt where H is the total enthalpy of material inside the control volume, T and Tw are temperatures of core and wall of mould respectively, Se represents effective thermal conductivity of steam in the gap hG and porous layer of crust h and thermal conductivity c (1/e=1/+h/c). The heat transfer coefficient =v/hG is fully determined by the thermal conductivity of steam v and by the thickness of gap hG. M v is the mass flow is the mass rate of vapour (evaporated bound and free water taken together), and M xs flow-rate of component x (water, solid) in the suspension flowing out of the control volume during expansion. cs, cw are specific thermal capacities of solid and water, rw is the latent heat. Eq. (1) can be rearranged into equation C A dT [Cs (1 A)Cc ] [ e S s ( M v M bc ) M v (1 A) c w ]( Tw T ) M v rw (2) dt M ws is the mass flow-rate of evaporated bound water from the crust, Cs and Cc where M bc heat capacity of suspension and core, respectively. To solve Eq.(2) the mass flow-rates of steam must be expressed in terms of temperatures T and Tw . The flow-rates are first related to the pressure drop across the porous crust pC=p-pG and along the surface in the gap pG=pG-pA , where p, pG, pA are pressures at the crust-core interface, at the surface and at the edge of sample, respectively (pA is in fact atmospheric pressure, because the calculated pressure drop in the venting channels of mould are negligible). Pressure drop pC is calculated from the Ergun’s equation for the flow resistance of a porous bed with porosity c, see Perry [6], 150 1 M v f M . p pG ( 175 . ) 3c M (3) c v Re c Dp v S 2 v pG was estimated from the simplified solution of radial laminar flow between parallel /S discs with a constant transversal mass flux M v 3 M v. pG p A v 3 v f G M hG (4) Additional pressure drops (venting channels, compact layer built at the crust surface, etc.) can be included into friction coefficient fG, too. The pressure p in Eq.(3) can be substituted by equilibrium temperature from the following approximation (5), (5) T 100m p , 0 where T is to be expressed in C and pressure p in bars (exponent m=4 for water). The only unknown quantity in Eq.(2) remains mass flow-rate of bound water. wall gap crust h core It is assumed that the intensity of evaporation is directly proportional to Temperature of bound the amount of bound water in the crust water increases and vapour tension p" too and to the difference between the partial pressure of bounded water p" p=p" vapour at a porous surface inside the p p"-p crust layer (this pressure is determined p Free water by temperature) and the pressure of Pressure decreases in flowing steam in the same place of the direction of flow pG crust (this pressure is determined by mb friction losses), see Fig.5. The mean pA value of this pressure difference is y estimated assuming linear temperature Bound water profile in the crust and cubic profile of mb(p"-p) pressure (this profile is determined uniquely knowing values of pressure Fig.5 Pressure and moisture profiles in crust and pressure gradients for y=0 and y=h). M p" p , M bc bc (6) where is a rate coefficient and exponent =1 was considered in simulations. Combining Eqs.(3-6) we could solve Eq.(2) for temperature T(t). However, the thickness of crust h, must be calculated too, because it determines overall heat capacity of the control volume and first of all the heat flux from the wall (this quantity is included into effective conductivity e). The time change of the thickness h is described by differential equation, following from the mass balance of the expanding core M bc dh M V V (7) v (1 ss bs ) dt S (1 s ) Vws and s is the porosity of core, related to the volumetric flowrate of suspension 16 ( H h) 2 (8) Vs ( p pA ) . 3S (1 s ) 2.b Implementation and simulation The model described above is a part of family of more than ten similar integral models, differing slightly by assumptions concerning expansion of material, bound water evaporation, core and gap resistance calculation. All these models were implemented on PC and intensively tested. These models are able to describe either temperature and pressure time courses exhibiting local maximum, see Fig. 6. Potato Starch: 40% - Thickness: 4 mm experimental predicted 200 180 Temperature [°C] 160 140 120 100 80 60 40 20 0 0 60 120 Time [s] 180 240 300 Fig.6 Temperature courses: experiment and numerical prediction of crust-core integral model CONCLUSIONS Baking process of starch-based materials depends significantly on the amount of bounded water and pressure of evaporated steam. Suggested integral model enables to describe temperature and pressure time courses having local minimum. It also enables to predict the baking time and its dependence on the thickness and other geometrical parameters of product. It allows for a more complete description of the process to be developed and an increased understanding of the parameters which affect product quality. List of most important symbols C heat capacity h hG thickness of crust thickness of gap at wall H M p rw S t T V half thickness of sample mass pressure latent heat of evaporation contact surface time temperature volume [J.K-1] [W.m-2.K1 ] [m] [-] porosity [m] thermal conductivity [W.m-1.K 1 ] [m] [Pa.s] viscosity of suspension [kg] rate coefficient of evaporation [-] [bar] Indices [J.kg-1] b bound water [m2] bc bound water in crust [s] c crust 0 [ C] s solid or suspension 3 [m ] v vapour w free water ws water suspension heat transfer coefficient References [1] Farkas B.E.: Heat and Mass Transfer in Frying. Journal of Food Engineering, 29 (1996) [2] Özilgen M., Heil J.R.:Mathematical Modeling of Transient Heat and Mass Transport in a baking Biscuit. J. Food Processing and Preservation, 18, pp.133-148, (1994) [3] Singh N., Akins R.G., Erickson L.E.: Modeling Heat and Mass Transfer during the Oven Roasting of Meat. Journal of Food Process Engineering, 7, pp. 205-220, (1984) [4] Singh R.P.: Heat and Mass Transfer in Foods during Deep-Fat Frying. Food Technology, pp. 134-137, (1995) [5] Zanoni B., Pierucci S., Peri C.: Study of the Bread Baking Process - II. Mathematical Modelling. Journal of Food Engineering, 23, pp. 321-336, (1994) [6] Perry R.H., Green D.W.: Chemical Engineers’ Handbook. McGraw-Hill, 1997 Acknowledgement: The project is supported by fund J04/98: 212200008