Higher SVR Rates With Peginterferon alfa-2a vs
advertisement

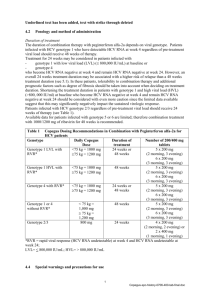
Peginterferon alpha-2a plus ribavirin versus peginterferon alpha-2b plus ribavirin in naive patients with chronic hepatits C virus infection: results of a prospective randomized trial. Posting Date: April 28, 2008 Prospective, randomized, single center, open-label study Summary of Key Conclusions Higher sustained virologic response (SVR) with peginterferon alfa-2a vs peginterferon alfa-2b therapy in treatment-naive chronic hepatitis C patients Effect largely observed in genotype 1 HCV patients with high HCV RNA Different response patterns in genotype 2 vs 3 suggest to evaluate these groups of patients separately Adverse effects similar between 2 groups Higher number of peginterferon alfa-2b patients discontinued treatment Background 2 well-accepted treatment options for chronic hepatitis C Peginterferon alfa-2a plus ribavirin Peginterferon alfa-2b plus ribavirin Efficacy comparisons of 2 peginterferon formulations only across studies to date Some pharmacokinetic differences have been identified Current study compared the efficacy and safety of peginterferon alfa-2a vs peginterferon alfa2b in treatment-naive HCV-infected patients Schematic of Study Design Eligibility Inclusion criteria > 18 years of age Detectable HCV RNA ALT > 1.5 x upper limit of normal within previous 6 months Liver biopsy within previous year Exclusion criteria Previous treatment experience HIV or HBV coinfection Other cause of chronic liver disease or any severe chronic disease History of decompensation Alcohol use within 6 months Severe hypertension Depression Neoplasia Active diabetes Pregnancy or lack of birth control use Hemoglobin < 12 g/dL Neutrophil count < 1.5 x 109/L Platelet count < 70 x 109/L Baseline Characteristics Characteristic Peginterferon alfa2a (n = 160) Peginterferon alfa2b (n = 160) 50.6 58.8 51.3 (10.3) 48.9 (11.3) 570 (0.37-8550) 604 (0.20-10800) 2.63 (1.76) 2.41 (1.32) 20.6 16.3 1 55.6 57.5 2 30.6 31.3 3 11.3 10.6 4 2.5 0.6 Male, % Mean age, yrs (SD) Median HCV RNA x 103 IU/mL (range) Mean ALT, x upper limit of normal (SD) Cirrhosis, % HCV genotype, % SD, standard deviation. Description of Current Analysis Intent to treat analysis Primary endpoint: SVR, defined as HCV RNA negative at 24 weeks after end of treatment Detection by qualitative PCR with sensitivity of 50 IU/L Patients also evaluated for end of treatment response Main Findings Higher SVR in peginterferon alfa-2a arm than peginterferon alfa-2b arm Greatest disparity in SVR rates among genotype 1/4-infected patients Trend toward higher SVR rates with peginterferon alfa-2a in patients with genotype 2 Similar rates with genotype 3 Higher rates of SVR with peginterferon alfa-2a in patients with high baseline HCV RNA Parameter, % Peginterferon alfa-2a (n = 160) Peginterferon alfa-2b (n = 160) P Value 68.7 54.4 .008 Genotypes 1/4 54.8 39.8 .04 Genotypes 2/3 88.1 74.6 .046 Genotype 2 91.8 77.8 .062 Genotype 3 77.8 70.6 .921 HCV RNA < 500,000 IU/mL 68.4 65.7 .73 HCV RNA ≥ 500,000 IU/mL 69.0 46.2 .002 Cirrhosis 42.4 46.2 NA 83.7 64.3 < .0001 Genotypes 1/4 75.3 49.5 < .0003 Genotypes 2/3 95.5 85.1 .04 SVR End of treatment response NA, not available. Peginterferon alfa-2a, male sex, absence of cirrhosis, and genotypes 2/3 significant predictors of SVR Other Outcomes Adverse effects similar between groups 18.7% of patients had anemia 2.5% of patients neutropenia 4.1% of patients had thrombocytopenia Higher number of peginterferon alfa-2b patients had treatment interruptions 14.0% and 13.4% of genotype 1/4 and 2/3 patients, respectively, treated with peginterferon alfa-2b discontinued therapy vs only 3.2% and 1.5% of patients, respectively, treated with peginterferon alfa-2a Reference Ascione A, De Luca M, Tartaglione MT et al. Peginterferon alpha-2a plus ribavirin versus peginterferon alpha-2b plus ribavirin in naive patients with chronic hepatits C virus infection: results of a prospective randomized trial. Program and abstracts of the 43rd Annual Meeting of the European Association for the Study of the Liver; April 23-27, 2008; Milan, Italy. Abstract 990.