Exam 2
advertisement

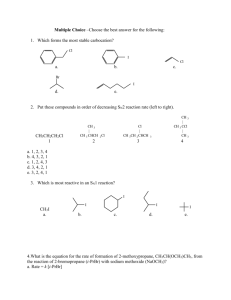
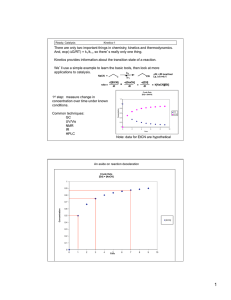
Exam 2 Chemistry 122 April 19, 2007 Do not open or begin this exam until instructed. This exam consists of 6 pages plus the cover page. Before starting the exam, check to make sure that you have all of the pages. The exam has a total of 100 points and includes 11 questions. Only legible answers written on the exam will be considered for grading. All pertinent information needed for the exam is given. Notes and textbooks are not permitted. Use your time wisely. This exam is administered under the Wake Forest Honor Code. Name_______________________________________ 1. (6 points, 2 each) Provide IUPAC accepted names for the following compounds. Br 2. (4 points, 2 each) Classify each of the following dienes as conjugated, cumulated, or isolated. 3. (2 points) Which of the following carbocations would be most likely to undergo a rearrangement? 4. (2 points) What is the oxidation state (or level) of the indicated carbon? O OH 5. (6 points) Draw a generic reaction energy diagram that fits the following profile: 1) the reaction is endothermic, 2) the reaction occurs in two steps mechanistically, and 3) the first step of the mechanism is rate determining. Be sure to label both axes. 1 6. (10 points, 2 each) Circle the reaction in each pair that would be faster. Br + NaCN + NaCN Br 25 oC CN DMSO + NaBr + NaBr 25 oC DMSO CN _________________________________________________________ Cl Cl + + NaCN NaCN 25 oC DMSO CN + NaBr CN + NaBr 25 oC ethanol _________________________________________________________ I OCH3 (+ CH3OH2 + I ) CH3OH I OCH3 (+ CH3OH2 + I ) CH3OH _________________________________________________________ Cl I + NaI + NaI DMSO Br + NaCl + NaBr I DMSO _________________________________________________________ Br + (CH3)3COK 80 oC + (CH3)3COH + KBr (CH3)3COH Br + (CH3)3COK 25 oC + (CH3)3COH + KBr (CH3)3COH _________________________________________________________ 2 7. a. (6 points) Draw the four -molecular orbitals of 1,3-butadiene. Show electrons in the orbitals that are filled. Label the HOMO and the LUMO. b. (3 points) Do the same for the two -molecular orbitals of ethene. c. (1 point) Would a reaction involving the HOMO of butadiene and the LUMO of ethene be symmetry allowed? energy ethene energy 1,3-butadiene 8. (36 points, 3 each) Provide structures for the major organic products of each of the following reactions. If more than one compound is expected, indicate which will be formed in greatest yield. Be careful to indicate product geometry when necessary. 1) BH3 2) H2O2, NaOH, H2O OTs + NaSCH2CH3 DMSO 3 O + O + OH HCl 1) OsO4 2) NaHSO3, H2O + HBr (1 equivalent) Li NH3 Br Br + CH3OH O Cl S O + OH H NaOCH3 (excess) pyridine 25 oC Cl CH3CH2OH 1) O3 2) Zn, H2O Br + NaOCH3 CH3OH 4 9. (8 points) Provide a complete electron-pushing mechanism for the following reaction. Include by-products as they are formed. Cl Cl2 CH3CH2OH H H OCH2CH3 10. (8 points) Provide a synthesis of 2-bromopentane. You may begin with any organic reagents containing three carbons or less and any inorganic reagents you need. 5 Choose one of the following problems. Clearly indicate which one you are omitting. 11. (8 points) Provide a complete electron-pushing mechanism for the following reaction. HBr Br H2O2 OR Provide a synthesis of the following target compound. You may beginning with any organic reagents containing four carbons or less and any inorganic reagents you need. O O H 6