Distribution of free amino acids, polyphenols and sugars
advertisement

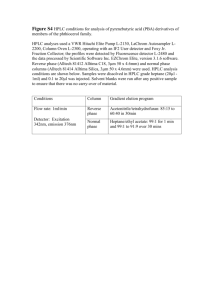
SUPPLEMENTARY MATERIAL Distribution of free amino acids, polyphenols and sugars of Ziziphus jujuba pulps harvested from plants grown in Tunisia M. Elaloui1*, A. Laamouri1, J. Fabre2, C. Mathieu2, G. Vilarem2 and B. Hasnaoui3 1 Institut National des Recherches en Génie Rural, Eau, Forêts (INRGREF), rue Hedi Karray, Elmenzeh IV, BP 10, 2080, Ariana, Tunisie tel: 71 709 033 Fax: (216) 71 717 951 2 Laboratoire de Chimie Agro-Industrielle (LCAI), UMR 1010 INRA- INPT/ENSIACET 4 allée Emile Monso 31030, Toulouse cedex, 4, France 3 Institut Sylvo-pastoral de Tabarka, 8110, Tabarka, Tunisie Abstract Ziziphus jujuba pulps are very much appreciated by the inhabitants and have been recently exported. The present paper reports on the pulps chemical composition (amino acids, polyphenols and sugars) of four Ziziphus jujuba ecotypes (Choutrana, Mahdia, Mahres and Sfax). These pulps with a nutritional interest should be promoted at least at a regional level. The major amino acids identified were the proline, the aspartique acid, the glutamic acid,. Among these, the proline was the most abundant amino acid (17.4 mol). Considerable differences total phenolic contents (15.85 mg/L) were found. Predominant phenolics analyzed with HPLC were rutin (1.09 mg/L) and chologenic acid (2.57 mg/100 g). Sugars isolated Ziziphus pulps were found at the rate of 43.52%. Using HPLC method, three sugars from pulps extract were identified: glucose, galactose and sucrose. The Mahdia ecotype was the richest in theses sugars with 0.45 mg/L, 136.51 mg/L and 113.28 mg/L respectively. Keywords: Ziziphus; pulps; ecotype; amino acids; plyphenol; sugar; HPLC. *e-mail: maryoumaa2000@yahoo.fr 1 Sampling Ripe fruits (Figure S1) were collected in September 2009 from different parts of tree cultivated in the experimental station of “Rouhia” (northwestern Tunisia; 35° 40’15.39” N; longitude 9° 0.3 - 15.29 E; altitude 636 m). Identified by Laamouri (2009) A voucher sample is deposited at the Herbario of National Institute for Research in Rural Engineering, Water and Forests (INRGREF) in Tunisia. [Figure S1 near here] The plant material was collected from eight trees of each ecotype Z. jujuba. Fruits were picked up during the period from September to November. They were transported to the laboratory of INRGREF where pulps were hand removed from fruits with knife and seeds were deggaged. Plant materiel were placed in plastic bags and stored in the freezer at -80 °C until chemical analysis that were done in ENSCIACET laboratory. Methods Reagents and standards All solvents used in our experiments: aceton, methanol Follin cicalteux, rutin, chlorogenic acids and sugar standards were purchased from Sigma Aldrich (Steinheim, Germany). Amino-Acid standard solutions were from Sigma-Aldrich, France. Buffer solutions are from Biochrom, United Kingdom. Amino acids analysis The amino-acid composition of the proteins was realized with an Ion Exchange Chromatography, according to the method of Moore and Stein 1948). The proteins were first hydrolyzed with hydrochloric acid. The samples were immersed with HCl 6N (10 mL for 100mg of proteins) in pyrex glass tubes. After nitrogen bubbling to remove oxygen from the sample, tubes were hermetically sealed and placed at 103°C for 24 hours. Samples are then dried with a rotary evaporator and diluted with a buffer at pH 2.2. After a filtration through a 0.45µm filter, a fixed amount of an internal standard, norleucine, was added to the samples. They were then analysed with the amino-acid analyzer Biochrom 20+ Biochrom Ltd. (Cambridge, UK). The device was 2 equipped with a system «column + pre-column» filled with sodium based ion exchange resins, 200 x 4.6 mm. The separation of the amino-acids was accomplished through an elution with different pH buffers at different column temperatures. After reaction with nihydrin in a reaction coil at 135°C, amino-acid were detected through visible absorption at a wavelength of 570nm or 440nm (for proline). Amino-acid standard solutions were also analysed to get calibration curves. A quantitative analysis of the samples can then be performed. The values obtained were validated through comparison with the internal standard concentration. Phenolic compounds Phenolic amounts The contents of total phenolic compounds of extracts from four ecotypes of Z. jujuba pulps, expressed as mg of gallic acid equivalents per gram of dry weight (mg GAE/g DW), were performed using gallic acid as a standard. So, dilutions from 0 mg / L to 100 mg / L. was prepared from gallic acid (2g / L) solution. An aliquot (0.125mL) of a suitable diluted methanolic fruit extract was added to 0.5mL of deionized water and 0.125mL of the Folin–Ciocalteu reagent. The mixture was shaken and allowed to stand for 6 min, before adding 1.25mL of 7% Na2CO3 solution. The solution was then adjusted with deionized water to a final volume of 3 mL and mixed thoroughly. After incubation for 90 min at 23C, the absorbance versus prepared blank was read at 760nm. The absorption was performed in triplicate using a SHIMADZU UV-1800 spectrophotometer. HPLC analysis The crushed Chinese jujube (2.5 mg) were macerated with acetone–water (4:1, v/v) at room temperature for 6 h. The acetonic extract was then filtered and a second maceration was applicated to the culot. The supernatants were combined and acetone was evaporated at 40°C under vacuum. The solution was passed through membrane filters with 0.45 mm pore size (Vivascience AG, Hanovre, Allemagne) before HPLC injection. Sugar analysis Ziziphus materials were extracted by macerating of 1 g of fresh pulp in 50 mL of UHQ water for 6 h. The macerate was centrifuged. The supernatant was filtrated using a Whatman filter paper type and kept in the freezer before HPLC analysis. 3 Analysis conditions Sugars analyses were performed by HPLC Dionex de type P199. A Carbo Pac PA1 capillary column (250 × 4 mm) was used. HPLC worked under suitable programs: In the first time it is stabilized to 1 Mm during 10min, then increased from 39°C to 43°C at 100 Mm, 43°C min−1 to 48°C at 100 Mm and 48 °C min−1 to 49 °C at 1 Mm. The carrier gas was helium with a flow rate of 1 ml min−1 (a post column injection was used). The injection volume was 25 µL. The identification of the compounds was done by comparison to the commercial standards. Column wash between two successive injections was made with methanol. Statistical analysis Data were subjected to a statistical analysis using the program package STATISTICA. Total volatile compounds are means ± SD of three experiments. The one-way analysis of variance (ANOVA) followed by Duncan multiple range test was employed. References Laamouri A., 2009. Contribution à l’étude des jujubiers en Tunisie : Identification, caractérisation, adaptation au déficit hydrique et multiplication. National Agronomic Institute. Tunisia. 4 Table S1: Sugars compositions of the pulps of four Tunisian Ziziphus jujuba ecotypes. Sugars Ecotypes Galactose Glucose Sucrose Mahdia 0,45 ± 0,00a 136,51 ± 1,18a 113,28 ± 2,56a Mahres 0,41 ± 0,01b 131,01 ± 0,7b 91,71 ± 1,13b Sfax 0,2 ± 0,00d 88,3 ± 1,97c 78,48 ± 2,64c Choutrana 0,36 ± 0,00c 115,4 ± 0,47d 91,08 ± 0,45b Figure S1. Fruits of Ziziphus jujuba (experimental station-rouhia) 5 a b Chromatogram S1. Free amino acids (a) and proline (b) of Z. jujuba pulps. 6 C18 column, injection volume = 20μl, flow = 0.8 ml / min, mobile phase = methanol/water3% acetic acid. Chromatogram S2. Pics detected in fruits extract of Ziziphus jujuba injected into the HPLC chain. Colonne = Carbo Pac PA1, volume injection = 25μl, flow = 1 ml/min. Chromatogram S 3. GC aqueous extracts (in mg / L) of soluble sugars existing solutions Ziziphus jujuba extract. 7