Lorazepam
advertisement

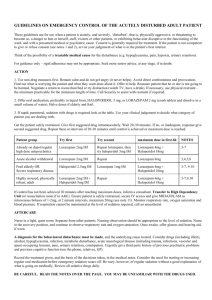
Name of Trust / logo Memo – shortage of supply To: From: Date: 31/7/13 Re: Shortage of lorazepam injection (Ativan®) 4mg/mL Description of product affected Lorazepam 4mg/mL injection (Ativan®), manufactured by Pfizer Ltd.. Background to shortage Pfizer are experiencing supply problems with Ativan (lorazepam) Solution for Injection 4mg/ml and do not anticipate that new stock will be available before September 2013 (1). Implications for patient care Lorazepam is a short-acting benzodiazepine. Lorazepam injection is licensed for the following indications (2): Pre-operative medication or premedication for uncomfortable or prolonged investigations for patients over 12 years e.g. bronchoscopy, arteriography, endoscopy The treatment of acute anxiety states, acute excitement or acute mania in patients over 12 years The control of status epilepticus for adults and children. Additionally, lorazepam injection is used outside license for sedation and agitation in ventilated adults and children, and as premedication in children. This document addresses the impact of the shortage of lorazepam injection on the management of these conditions. Alternative agents and management options Listed below are potential alternative agents that might be considered for use in the absence of a licensed version of lorazepam injection. For some of the clinical indications discussed consideration should be given to the importation of unlicensed lorazepam injection as there is not a directly suitable alternative agent available*. 1. Status epilepticus: In the hospital setting, advice from the NICE guideline on the management of epilepsies suggests the use of intravenous lorazepam 100 micrograms/kg (usually a 4 mg bolus, repeated once after 10-20 minutes) first line (3). For neonates, a dose of 100micrograms/kg as a single dose (repeated once if initial dose is ineffective) is used, and for children aged 1 month to 12 years, a dose of 100 micrograms/kg, to a maximum of 4mg is used, repeated once if the initial dose is ineffective (4). Alternatives to intravenous lorazepam are rectal diazepam and buccal midazolam (paediatric license only) Rectal diazepam 500micrograms/kg up to a maximum of 30mg (elderly patients should receive only 250micrograms/kg up to a maximum of 15mg) (5). The following rectal diazepam doses are recommended for children (4): Neonate: 1.25-2.5mg repeated after 10 minutes if necessary Child 1 month to 2 years: 5mg repeated after 10 minutes if necessary Child 2 to 12 years: 5-10mg repeated after 10 minutes if necessary Child 12 to18 years: 10mg repeated after 10 minutes if necessary However, midazolam given by the buccal route, as a single dose of 10mg, may be more acceptable to some patients, (5). The following buccal midazolam doses are recommended for children (4): Neonate: 300micrograms/kg repeated once after 10 minutes if necessary Child 1 to 3 months: 300micrograms/kg (max 2.5mg) repeated once after 10 minutes if necessary Buccolam prefilled syringes are licensed from 3months – 18 years Child 3 months to 1 year: 2.5 mg repeated once after 10 minutes if necessary Child 1 to 5 years: 5mg repeated once after 10 minutes if necessary Child 5 to 10 years: 7.5mg repeated once after 10 minutes if necessary Child 10 to 18 years: 10mg repeated once after 10 minutes if necessary Midazolam or diazepam may also be used intravenously (please refer to the BNF for Children for dosing information). 2. Treatment of acute anxiety states, acute excitement or acute mania or acutely disturbed or violent behaviour: According to the Drug and Therapeutics Bulletin review on drugs in the doctors’ bag, acute management of anxious, agitated or psychotic patients should initially involve establishing a good rapport and attempting to calm them down. The review states that when medication for reducing anxiety or agitation is considered necessary, it should be given by mouth wherever possible, rather than by injection. If parenteral treatment is necessary, either haloperidol 210mg intramuscularly (5mg/mL injection) or lorazepam 1-2mg intramuscularly (4mg/mL injection) may be used (5). It should be noted that subsequent to publication of this advice, ECG monitoring (to measure the QTc interval) is now mandatory for haloperidol (both oral and IM). Personal communication with staff at the Maudsley Hospital indicates that they now use no more than 5mg of haloperidol by intramuscular injection for this indication. For patients who are not already on, or have not previously been treated with an antipsychotic, benzodiazepines should be used first line, to avoid dystonic reactions with parenteral antipsychotics (6). In circumstances when an intramuscular injection of a benzodiazepine is needed lorazepam is viewed as the agent of choice and stocks should be prioritised for this indication (6). The South London and Maudsley Prescribing Guidelines suggest that for acutely disturbed or violent behaviour, oral treatment with lorazepam 1-2mg is an option if the patient is already taking a regular antipsychotic. An oral antipsychotic such as olanzapine, risperidone or haloperidol (with promethazine and QTc monitoring) can be considered in patients not already taking a regular oral or depot antipsychotic. Buccal midazolam (10-20mg) may also be considered at this stage (although unlicensed) to avoid the need to move on to an intramuscular injection (7). If oral/ buccal treatment is not appropriate, or if two doses fail (with 45-60 minute gap between doses), then intramuscular treatment with any of the following drugs should be considered depending on clinical circumstance (7). lorazepam 1-2mg promethazine 50mg olanzapine 10mg aripiprazole 9.75mg Haloperidol (5mg) can also be used but should be the last drug considered because of the relatively high incidence of acute dystonia and the recommendation that patients should have a pre-treatment QTc monitoring. It is also recommended that following any parenteral drug administration in these circumstances, temperature, pulse rate, blood pressure and respiratory rate should be monitored every 5-10 minutes for 1 hour, and then every 30 minutes until the patient is ambulatory (7). 3. Pre-operative medication or premedication for uncomfortable or prolonged investigations The British Society of Gastroenterologists guidance on “Safety and sedation during endoscopic procedures”, recommend the lowest possible dose of benzodiazepines for sedation during endoscopic procedures (8). The guidance also specifies that 5 mg of midazolam should usually be the maximum dose given and that elderly patients are given 1-2 mg initially with a sensible pause to observe effect. For premedication in children, the BNF for Children lists oral midazolam as the most common premedicant for children (can also be given rectally or by IV injection) and alternatives include oral temazepam in older children, chloral hydrate and the antihistamine alimemazine. (4). As these are unlicensed indications in children, please refer to the relevant monographs for dosing information, and to local practice guidelines if more appropriate. 4. Sedation in ventilated patients (unlicensed use): For ventilated adult patients, enteral lorazepam can be used in the majority of patients. Lorazepam tablets may be crushed and administered via the nasogastric tube. Sublingual administration has also been used successfully on a number of occasions. Midazolam may also be used as an alternative, but both midazolam and its active metabolite may accumulate in critical illness, which may lead to prolonged sedation, particularly in cardiothoracic patients and in those with globally impaired organ dysfunction. For children, morphine, diazepam, intravenous clonidine, choral hydrate, or midazolam may be used (4, 10). As these medicines may be unlicensed for this indication in children, please refer to the relevant monographs for dosing information, and to local practice guidelines if more appropriate. Disclaimer: The content of some of this memo is based on consensus opinion from clinical practitioners. Users should bear this in mind in deciding whether to base their policy on this document. Individual trusts should ensure that procedures for unlicensed medicines are followed where a foreign import drug is required.* References: 1. Personal Communication – Department of Health (July 2013) 2. Ativan SPC. Wyeth Pharmaceuticals. Date of revision May 2012 3. NICE CG20 The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care - Full guideline http://www.nice.org.uk/pdf/CG020fullguideline.pdf 4. British Medical Association, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatrics and Child Health, and, Neonatal and Paediatric Pharmacists Group. BNF for Children 2013-14. 5. Drugs for the doctor’s bag: Adults. Drug and Therapeutics Bulletin September 2005; 43: 65-72. 6. Personal communication – Anne Connolly, Principal Pharmacist, Medicines Information, South London and Maudsley NHS Trust 7. The South London and Maudsley NHS Trust and Oxleas NHS Trust Prescribing Guidelines (11th edition) 8. British Society of Gastroenterology. Safety and Sedation During Endoscopic Procedures. http://www.bsg.org.uk/pdf_word_docs/sedation.doc 9. Personal communication - Catherine McKenzie, Principal Pharmacist, ICU Critical Care, Guy’s and St Thomas’ NHS Foundation Trust. 10. Guy’s and St Thomas’, King’s College and University Lewisham Hospitals Paediatric Formulary, 9th edition 11. Personal communication – William Thornhill, Senior Paediatric Pharmacist, Evelina Childrens Hospital, Guys and St Thomas’ NHS Foundation Trust. Original document prepared by: Hina Radia Guy’s and St Thomas’ NHS Foundation Trust Medicines Information Centre 1 December 2005 and updated by David Erskine in June 2010 and July 2013 Document modified by: Name of individual at other centre using the product with modifications, centre, date For all correspondence please contact: Name of person at base hospital where memo is circulated (i.e. NOT the original author at Guy’s and St Thomas’ NHS Foundation Trust