1 SUPPLEMENTARY MATERIAL ABBREVIATIONS cTFC
advertisement

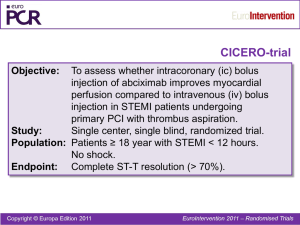
1 SUPPLEMENTARY MATERIAL ABBREVIATIONS cTFC= corrected TIMI frame count EMP = endothelial derived microparticles MBG = myocardial blush grade PMP = platelet derived microparticles pPCI = primary percutaneous coronary intervention PPP = platelet poor plasma QuBE = quantitative blush evaluator TS = thrombus score ΣSTR = ST-Segment Resolution 2 SUPPLEMENTARY METHODS Angiographic analysis of thrombotic burden Thrombus score (TS) 0 corresponded to no angiographic evidence of thrombotic material; TS 1 corresponded to possible thrombus, appearing as a convex, hazy lesion with irregular contours at the site of total occlusion; TS 2 corresponded to definite thrombus ≤ 1/2 the vessel diameter; TS 3 corresponded to definite thrombus > 1/2 but < 2 vessel diameters; thrombus grade 4 corresponded to definite thrombus ≥ 2 vessel diameters; TS 5 corresponded to inability of thrombus burden assessment due to persistent total occlusion of the culprit vessel after guidewire passage. (1) Corrected TIMI frame count (cTFC) cTFC was measured at the end of pPCI by counting, in a final long angiographic run at 30 frame/second, the number of cineframes required for contrast to first reach standardized distal coronary landmarks in the infarct-related artery starting from the frame in which the dye fully enters the artery producing a column of nearly full dye across the entire width of the origin of the artery with antegrade motion (2). We used a power-injector (Avanta, Medrad Inc., Indianola, PA, USA) with injection of 6 ml of Iomeprol (Iomeron 350, Initios Medical AB, Goteborg, Sweden) at rate of 4 ml/sec and with a pressure limit of 300 psi for both left and right coronary arteries. Myocardial Blush Grade (MBG) MBG was scored as follows: 0, no myocardial blush or contrast density or persisting myocardial blush (“staining”); 1, minimal myocardial blush or contrast density; 2, moderate myocardial blush or contrast density but less than that obtained during angiography of a contralateral or ipsilateral non–infarct-related coronary artery; and 3, normal myocardial blush or contrast density, comparable 3 with that obtained during angiography of a contralateral or ipsilateral non–infarct-related coronary artery. (3) Quantitative Blush Evaluator (QuBE) At the end of each pPCI procedure, a long final run was acquired with the same settings used for MBG assessment, with the only exception of frame-rate acquisition that was modified at 15 frames/second. QuBE score was calculated offline by a separate observer blinded to clinical data. A manual region-of-interest was drawn in the vascular perfusion bed of the infarct-related artery, and the translational movement of the heart was manually corrected on each frame. The QuBE algorithm performs an automatic pixel analysis, by filtering all large-scale structures, dividing all the selected pixels in blocks and, considering the average of the darkest few pixels in each block, calculating the QuBE value as the average of the best 50% of pixel blocks. This process is repeated for 125 frames (8.3 seconds of acquisition at 15-frames/second), and a perfusion curve is generated. The QuBE score, expressed in arbitrary units, equals the maximum increase plus the maximum decrease in this curve. This methodology has been described in detail elsewhere and the software released as open-source code with explicit permission for other groups to use, redistribute and modify (4, 5). ST-Segment Resolution (ΣSTR) Each electrocardiogram was analyzed by a blinded assessor and summed ST-segment elevation was calculated as the sum of elevation in V1–6, I, and aVL for anterior infarction and as the sum of elevation in leads II, III, aVF, V5, and V6 for non-anterior infarction. ΣSTR was defined as the percent reduction in the summed ST-segment elevation score between electrocardiograms obtained prior to PCI and at 90 minutes. ΣSTR was classified as complete (≥70%), partial (30 to 70%), and absent (< 30%). (6) 4 Microparticles levels measurement All blood samples were collected in citrate-buffer tubes. All samples were analysed on-line immediately after the collection in the catheterization laboratory (time interval from blood sample collection to completed sample processing: 40±20 minutes; time for flow cytometry: 15±5 minutes) in order to prevent any sort of bias deriving from freezing and/or storing (7). In order to avoid platelet contamination, platelet-poor plasma (PPP) was obtained by centrifuging blood samples at 1500g for 15 minutes at room temperature and then by immediately centrifuging the supernatant at 13000g for 2 minutes. (8) For platelet derived microparticles (PMP) and endothelial derived microparticles (EMP) detection, 50 µl of PPP were incubated for 20 minutes at room temperature in the dark with 5 µl of PE -conjugated monoclonal antibody against CD31 (clone 1F11, cat 2409, Beckman Coulter, Miami, USA) and 5 µl of FITC-conjugated monoclonal antibody against CD42b (clone SZ2, cat PN IM0648U, Beckman Coulter, Miami, USA). EMP were defined as particles positively labelled for CD31 and negatively for CD42b (CD31+/CD42-), while PMP were defined as particles positively labelled for CD31 and CD42b (CD31+/CD42b+). 5 µl of appropriate fluorochrome-conjugated isotype-matched mAb were used as control for background staining (IgG1-FITC, clone 679.1MC7, cat A07795, Beckman Coulter, Miami, USA; IgG1-PE, clone 679.1MC7, cat A07796, Beckman Coulter, Miami, USA). After incubation, samples for PMP and EMP detection were resuspended in 500 µl of PBS and analyzed in a EPIC XL-MCL (Beckman Coulter, Miami, USA) flow cytometer using EXPO32 software (Beckman Coulter, Miami, USA); forward scatter and sideward scatter were set on a logarithmic gain, to best cover a wide size range. In order to assess the power of our cytometer in discriminating between 0.5 µm and 0.9 µm events (now considered mandatory before starting a microparticles measurement protocol by a recent consensus conference), (9) and to evaluate whether our results were comparable to those from other studies, standardization was achieved using a blend of monodisperse fluorescent beads (Megamix, BioCytex) of three diameters (0.5, 0.9 and 3 µm). Forward scatter (FS) and side scatter (SS) 5 parameters were plotted on logarithmic scales to best cover a wide size range. Single staining controls were used to check fluorescence compensation settings and to set up positive regions. (10) To enumerate MP, EPIC XL-MCL flow cytometer was set at “low” rate, and actual flow rate was calculated using Trucount beads (BD Biosciences) in an initial series of 20 samples. Consistency was checked monthly. The mean flow rate was 10.0 µl/min, with a variation coefficient of 5.04%. The volume sample analyzed was calculated with the formula: 10/60*T, where T was the time of analysis expressed in seconds, 10 was the calculated mean volume in µl analyzed by the cytometer in the unit of time (1 minute) and 60 was the conversion factor from minutes to seconds. 2500 events were collected and acquired. The time necessary for counting 2500 events was determined and MP concentration calculated as: (absolute number per microliter, n/µl) (n/l) = (Number of events/Volume sample analyzed)*(Total volume of the sample/Amount of PPP), where the total volume of the sample was 500 µl, and 50 µl was the amount of PPP. (11) Sample collection validation protocol To exclude that differences in MP levels from intracoronary and aortic samples could be related to collection strategy (guiding catheter vs thrombectomy device), ten additional patients with admission diagnosis of Non ST Elevation Myocardial Infarction (mean age 67±14 years) undergoing PCI were enrolled, and, after informed consent, MP levels in blood samples collected from the ascending aorta before PCI were compared. Briefly, with the guiding catheter still in the ascending aorta, a first sample of 30 ml was obtained from the guiding catheter lumen, then the thrombectomy device was inserted and, without exiting the guiding catheter tip, a further 30 ml were obtained from its lumen. 6 SUPPLEMENTARY RESULTS Microparticles, microvascular obstruction and thrombus score Intracoronary PMP levels continued to be related to MVO after correcting for TS. In higher TS classes, the presence of combined angiographic and electrocardiographic MVO (MBG< 2 and absent ST resolution) was significantly related to higher levels of intracoronary PMP and EMP. Indeed, intracoronary PMP were 5238.0 (3726.9-7924.7) vs 2747.2 (2678.8-4688.1), p: 0.04 for TS 5; 1061.3 (686.2-2249.0) vs 725.5 (258.2-898.7), p:0.02 for TS 4; 155.1 (84.0-230.9) vs 117.4 (77.4-245.5), p: 0.14 for TS 3; 32.2 (5.9-67.4) vs 41.5 (4.0-94.4) p: 0.32 for TS 0/1/2. The same trend was observed for intracoronary EMP : 1050.0 (763.6-2731.8) vs 530.5 (513.5-806.3) p: 0.02 for TS 5; 419.9 (48.4-1431.2) vs 322.5 (45.3-781.3) p: 0.04 for TS 4; 136.7 (85.0- 613.5) vs 102.3 (74.9-134.1) p: 0.09 for TS3; 73.5 (70.0-125.0) vs 70.2 (65.4-93.2) p: 0.10 for TS 0/1/2, but not for aortic PMP and EMP [Supplementary Figure 3]. Effect of blood sampling by thrombus aspiration device on aortic microparticles levels No significant differences were observed between both PMP and EMP levels in blood samples collected from ascending aorta by guiding catheter and by thrombectomy device (161.0 (119.0328.7) vs 172.5(125.7-344.2) respectively, p: 0.13 for PMP and 88.5 (68.5-109.0) vs 85.7 (73.7102.0) respectively, p: 0.78 for EMP). Moreover, both PMP and EMP levels in blood samples obtained with the two collection methods were strongly related (r: 0.9; p<0.001 for both aortic PMP and EMP) [Supplementary Figure 4]. 7 SUPPLEMENTARY MATERIAL REFERENCES 1. Sianos G, Papafaklis MI, Serruys PW. Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol. 2010;22:6B-14B. 2. Gibson CM, Cannon CP, Daley WL, Dodge JTJ, Alexander BJ, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, Braunwald E. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879-88. 3. van 't Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. 1998;97:2302-6. 4. Vogelzang M, Vlaar PJ, Svilaas T, Amo D, Nijsten MW, Zijlstra F. Computer-assisted myocardial blush quantification after percutaneous coronary angioplasty for acute myocardial infarction: a substudy from the TAPAS trial. Eur Heart J. 2009;30:594-9. 5. Haeck JD, Gu YL, Vogelzang M, Bilodeau L, Krucoff MW, Tijssen JG, De Winter RJ, Zijlstra F, Koch KT. Feasibility and applicability of computer-assisted myocardial blush quantification after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Catheter Cardiovasc Interv. 2010;75:701-6. 6. Sorajja P, Gersh BJ, Costantini C, McLaughlin MG, Zimetbaum P, Cox DA, Garcia E, Tcheng JE, Mehran R, Lansky AJ, Kandzari DE, Grines CL, Stone GW. Combined prognostic utility of ST-segment recovery and myocardial blush after primary percutaneous coronary intervention in acute myocardial infarction. Eur Heart J. 2005;26:667-74. 7. Shah MD, Bergeron AL, Dong JF, Lopez JA. Flow cytometric measurement of microparticles: pitfalls and protocol modifications. Platelets. 2008;19:365-72. 8 8. Robert S, Poncelet P, Lacroix R, Arnaud L, Giraudo L, Hauchard A, Sampol J, Dignat- George F. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies? J Thromb Haemost. 2009;7:190-7. 9. Lacroix R, Robert S, Poncelet P, Kasthuri RS, Key NS, Dignat-George F; ISTH SSC Workshop. Standardization of platelet-derived microparticle enumeration by flow cytometry using calibrated beads: results of ISTH SSC collaborative workshop. J Thromb Haemost 2010; 8: 25712574. 10. Megamix Bead Manufacturer's Instructions http://www.milananalytica.ch/_downloads/spec_sheet/7801MegaMix/MegaMix_package_inse rt.pdf. (27 November 2011) 11. van Ierssel SH, Van Craenenbroeck EM, Conraads VM, Van Tendeloo VF, Vrints CJ, Jorens PG, Hoymans VY. Flow cytometric detection of endothelial microparticles (EMP): Effects of centrifugation and storage alter with the phenotype studied. Thromb Res. 2010;125:332-39. 9 SUPPLEMENTARY FIGURES Figure 1. Study Flow Chart. The figure summarizes the main steps for selection of patients included in the study. (pPCI = primary percutaneous coronary intervention, MP= microparticles, pts= patients, STEMI= ST elevation myocardial infarction) Figure 2. Correlations of intracoronary and aortic PMP and EMP levels and cTFC and QuBE score. Scatter plots show the relationship between intracoronary and aortic PMP and EMP levels with continuous indexes of MVO. Higher MP levels are related to higher cTFC and lower QuBE values. Data are presented as scatter plot. R= Person's correlation coefficient. Figure 3. Relationship between intracoronary PMP and EMP and combined electrocardiographic (incomplete ΣSTR) and angiographic (MBG <2) MVO according TS classes. Figure clearly shows how in higher TS classes both intracoronary PMP and EMP are related to increased occurrence of combined MVO, defined as a combination of incomplete ΣSTR and MBG < 2. (* stands for p < 0.05) Figure 4. Relationship between PMP and EMP in blood samples from ascending aorta obtained by guiding catheter and thrombus aspiration device (Diver CE Max, Invatec, Italy). Scatter plots describe the strong relationship between PMP and EMP levels in blood samples collected from ascending aorta by two different sampling strategies: guiding catheter versus thrombus aspiration device. (Data are presented as scatter plot. R= Person's correlation coefficient). PMP: platelet derived microparticles EMP: endothelial derived microparticles MBG: myocardial blush grade ΣSTR: ST resolution MVO: microvascular obstruction TS: thrombus score QuBE: quantitative blush evaluator 10 cTFC: corrected TIMI frame count