Ch15
advertisement
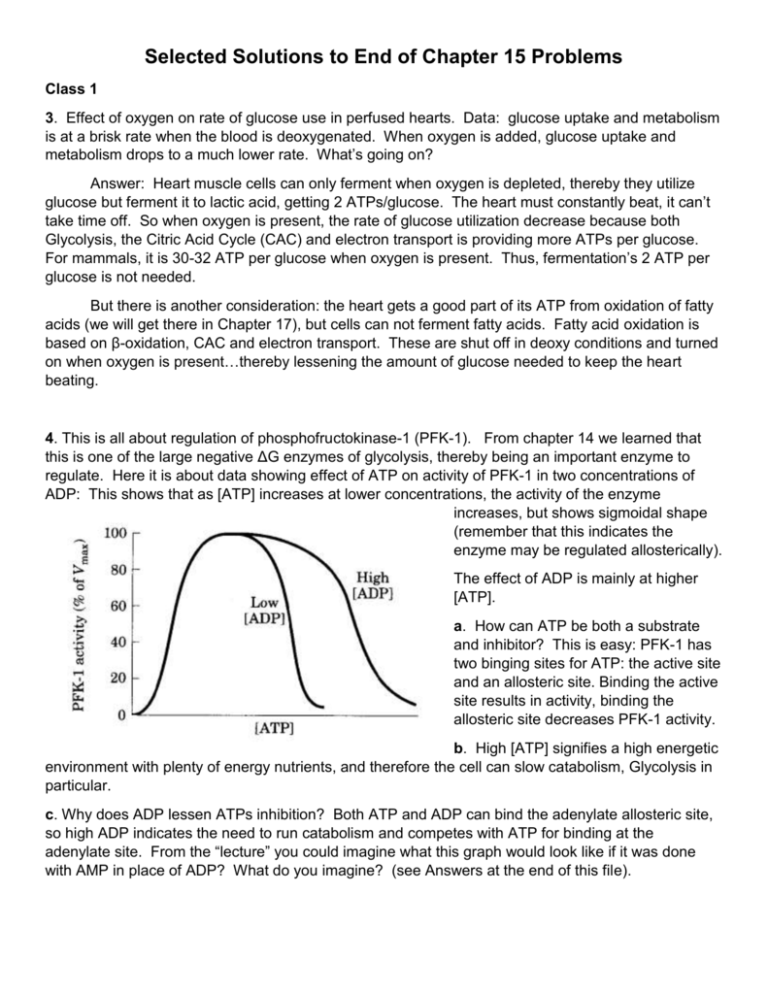
Selected Solutions to End of Chapter 15 Problems Class 1 3. Effect of oxygen on rate of glucose use in perfused hearts. Data: glucose uptake and metabolism is at a brisk rate when the blood is deoxygenated. When oxygen is added, glucose uptake and metabolism drops to a much lower rate. What’s going on? Answer: Heart muscle cells can only ferment when oxygen is depleted, thereby they utilize glucose but ferment it to lactic acid, getting 2 ATPs/glucose. The heart must constantly beat, it can’t take time off. So when oxygen is present, the rate of glucose utilization decrease because both Glycolysis, the Citric Acid Cycle (CAC) and electron transport is providing more ATPs per glucose. For mammals, it is 30-32 ATP per glucose when oxygen is present. Thus, fermentation’s 2 ATP per glucose is not needed. But there is another consideration: the heart gets a good part of its ATP from oxidation of fatty acids (we will get there in Chapter 17), but cells can not ferment fatty acids. Fatty acid oxidation is based on β-oxidation, CAC and electron transport. These are shut off in deoxy conditions and turned on when oxygen is present…thereby lessening the amount of glucose needed to keep the heart beating. 4. This is all about regulation of phosphofructokinase-1 (PFK-1). From chapter 14 we learned that this is one of the large negative ΔG enzymes of glycolysis, thereby being an important enzyme to regulate. Here it is about data showing effect of ATP on activity of PFK-1 in two concentrations of ADP: This shows that as [ATP] increases at lower concentrations, the activity of the enzyme increases, but shows sigmoidal shape (remember that this indicates the enzyme may be regulated allosterically). The effect of ADP is mainly at higher [ATP]. a. How can ATP be both a substrate and inhibitor? This is easy: PFK-1 has two binging sites for ATP: the active site and an allosteric site. Binding the active site results in activity, binding the allosteric site decreases PFK-1 activity. b. High [ATP] signifies a high energetic environment with plenty of energy nutrients, and therefore the cell can slow catabolism, Glycolysis in particular. c. Why does ADP lessen ATPs inhibition? Both ATP and ADP can bind the adenylate allosteric site, so high ADP indicates the need to run catabolism and competes with ATP for binding at the adenylate site. From the “lecture” you could imagine what this graph would look like if it was done with AMP in place of ADP? What do you imagine? (see Answers at the end of this file). 5. The blood glucose concentration is stated to be 5 mM, actually from individual to individual it varies between 3 to 6 mM. Why is the concentration of glucose in cells less than 5 mM? As soon as glucose enters the cell, it is immediately phosphorylated by hexokinase and is no longer glucose because it is glucose-6-phosphate that can enter the Glycolysis, the Pentose-Phosphate-Pathway or Glycogen synthesis. The last part asks if it would be useful to give IV administration of glucose-6-phosphate. The answer is a big NO. It is only glucose that can get into a cell is by a glucose transporter, glucose-6phosphate would not at all be transported into the cell and certainly not at all by diffusion. Class 2 6. Enzyme activity and physiological function problem. Data: the Vmax of glycogen phosphorylase is much greater in the muscle enzyme than the liver enzyme. When a question like this is posed, it is not asking for kcat but rather regulation between inactive (b and T states) and active (a and R states). a. What is the physiological function of each glycogen phosphorylase? In muscle it is used only to provide glucose for catabolism to make ATP for muscle activity. Most of the time skeletal muscles are at rest and glycogen phosphorylase is in the T state. AMP activates muscle glycogen phosphorylase to activity and a high kcat. In the liver, glycogen phosphorylase is there to maintain blood glucose levels which normally do not vary dramatically. It needs to be turned off when blood glucose is high, an on when blood glucose is low. b. Muscle enzyme needs a higher Vmax for strenuous muscular activity, particularly when the muscle is working under very reduced oxygen concentrations. Glycogen phosphorylase in the liver is an isozyme of the muscle enzyme, and regulated by glucose (inhibitor) as well as by hormones. 7. Glycogen phosphorylase equilibrium. Data: ΔGo’ of glycogen phosphorylase is 3.1 kJ/mole. The glycogen phosphorylase reaction: Glycogenn + Pi Glycogenn-1 + glucose-1-phosphate a. Calculate the ratio of [Pi] to [glucose-1-phosphate] at equilibrium (note that we are not using the [glycogen] or [glycogen – 1 glucose] because glycogen is so large the ratio of [glycogen]/[glycogen-1 glucose] is always very close to one: ΔGo’ = - RT ln Keq rearrange to ln Keq = - ΔGo’ / RT ln Keq = - (3.1 kJ/mole) / (2.48 kJ/mole) = -1.25 Keq = e-1.25 = 0.29 so this is the ratio of [glucose-1-phosphate] / [Pi] So the ratio of [Pi] / [glucose-1-phosphate] is the inverse of the Keq: 1 / 0.29 = 3.5 b. The ratio of [Pi] / [glucose-1-phosphate] is 100 in myocytes is high compared to the equilibrium value. This means in muscle the [glucose-1-phosphate] is very low and therefore pulls the glycogen phosphorylase reaction. 8. Glycogen phosphorylase regulated by covalent modification under different conditions: a. Treatment with ATP and phosphorylase kinase converts phosphorylase-b to phosphorylase-a by the addition of a phosphate to the protein making it the most active form glycogen breakdown increases. b. Threatment with phosphorylase phosphatase (a protein phosphatase) converts phosphorylase-a to phosphorylase-b by removal of a phosphate from the “a” form. This seriously slows glycogen phosphorylase. c. Addition of epinephrine to muscle cells causes the synthesis of c-AMP that activates Protein Kinase-a which phosphorylates glycogen phosphorylase kinase to add phosphates to glycogen phosphorylase thereby activating it to the “a” form. 10. Migrating birds travel thousands of miles. Other birds, rely on short intense flights to escape predators. Why must the regulation of energy supply of these two types of birds be different? Migrating birds must fly long distances, they need an energy supply that can be used slowly but has a high ATP production value. They use lipids, primarily fatty acids which have carbons at their most reduced state: methyls which are also light compared to glycogen and the waters that are part of glycogen granules (but not fat). Utilization of fat is a slower, but energy rich process which is perfect for migrating birds. Using glycogen is faster and the birds run out of it in a short time which is perfect for escaping predators (or not). 15. Between dinner and breakfast your blood glucose drops and the liver becomes the major producer of glucose rather than a consumer of glucose. What are the hormonal basis for the switch from consumer of glucose and then producer of glucose? During and shortly after dinner, the pancreas produces insulin to increase liver glucose consumption and storage as glycogen. As glucose levels begin to decrease, the pancreas stops producing insulin and begins to produce glucagon (both are peptide hormones). Remember glucagon means “glucose gone”. Glucagon is sensed by G protein coupled receptors which activates glycogen phosphorylase (cAMP activation of protein kinase A activating glycogen phosphorylase kinase that phosphorylates glycogen phosphorylase-b converting it to glycogen phosphorylase-a that can convert glycogen glucoses to glucose-1-phosphate. Remember from “lecture” how liver cells get glucose exported to blood. Glucagon also stimulates gluconeogenesis by stimulating fructose-1,6-bisphosphatase (by lowering [fructose-2,6-bisphosphate] and inhibiting glycolysis. Liver cells in these conditions can supply themselves with energy from amino acids and fatty acids (this is coming in Chapters 17 and 18). 16. Genetic knock outs in mice. Genetic manipulation of mice can remove a functional gene, that gene has been knocked out. After that, what are the metabolic consequences? Here are some knock outs that are not lethal, predict what the metabolic consequences are (do only a through e): a. knock out glycogen debranching enzyme. b. knock out hexokinase-IV in the liver. c. knock out FBPase-2 in liver. d. knock out a protein that makes FPBase-2 constitutive (on all the time). e. knock out a protein that makes muscle AMPK constitutive. Answers: a. reduced capacity to use glycogen (incomplete glycogen breakdown): can’t fully produce glucose between meals, blood glucose goes very low levels. b. can not utilize blood glucose (can not form normal glycogen levels), high blood glucose after meals. c. higher levels of fructose-2,6-bisphosphate, stimulating glycolysis and shutting down gluconeogenesis. d. reduced fructose-2,6-bisphosphate, inhibiting glycolysis and stimulating gluconeogenesis. e. increased uptake of glucose and fatty acids oxidation of both. Answer to question posed in question 4: AMP works even better being the major indicator of low energy charge. In the graph, the effect of ATP would be the same at low concentrations and continue beyond the ADP effect, this is because AMP binding to the adenylate site is stronger than ATP and ADP.









