CHEM 322L Instrumental Analysis
advertisement

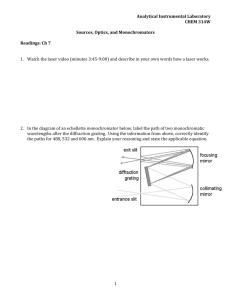
CHEM 322L INSTRUMENTAL ANALYSIS LAB SYLLABUS Dr. DiLella Spring 2011 DESCRIPTION CHEM 322L is a one-credit laboratory course that provides hands-on experience using modern analytical instrumentation and techniques. Students learn how to prepare samples for and how to operate several types of instruments. Topics covered include atomic spectroscopy, molecular spectroscopy, electrochemistry, mass spectroscopy and chromatography. Students are introduced to and are expected to use advanced data handling and error treatment methods. Personal computers are available for use in data analyses. Students are expected to use a computer spreadsheet program for data analysis and presentation. TEXT Handouts OFFICE HOURS MWF 10:10-11:00, MW 3:10-4:00, R 1:10-2:00, F 12:10 – 1:00 or by appointment Office : Byrd Center 315 304-876-5430 ddilella@shepherd.edu web page: http://WEBPAGES.SHEPHERD.EDU/DDILELLA/ CLASS HOURS R 9:35 - 12:25 PREREQUISITES CHEM 209, 209L are required. CHEM 321 and CHEM 321L are strongly recommended. CHEM 322 should be taken concurrently Computer skills are not a prerequisite but students who do not know how to do basic computer spreadsheet operations should see the instructor as soon as possible to get special tutoring in these skills. With the aid of a computer spreadsheet program, the time required to do the lab reports will be significantly reduced. SAFETY The experiments have been designed with safety as a major consideration. If everything went as planned there would be little danger. However, the equipment and procedures will be unfamiliar and mistakes can and will occur. Be sure that you understand the hazards associated with the equipment before starting an experiment. Do not be afraid to ask questions. SAFETY GOGGLES MUST BE WORN AT ALL TIMES. Students may NEVER work in a lab without permission of the instructor or without supervision. PREPARATION BEFORE THE LABORATORY You will be able to work much more efficiently in the lab if you understand what it is you are trying to do. READ THE TEXT BEFORE THE EXPERIMENT. Figure out how to prepare the solutions before coming to lab so you don't waste valuable lab time. Be prepared. The time spent in preparation is time that you won't have to spend in the lab. LAB NOTEBOOK 1 CHEM 322L INSTRUMENTAL ANALYSIS LAB SYLLABUS Dr. DiLella Spring 2011 All data should be entered in a bound lab notebook. Spiral bound notebooks are not acceptable. The lab notebook should include all information that was not known before the experiment. Each page should include the date and the name of the experiment. All entries should be clearly labeled and should have correct units. All measurements should include estimated uncertainties. Your notebook should be signed by the instructor at the end of each lab period. FINAL GRADE The final grade will be based solely on the lab reports. There will be no tests or quizzes. The final grade will be based on the following scale A B C D 90 % 80 % 70 % 60 % to 100 % to 89+ % to 79+ % to 69+ % LAB REPORTS Each lab report will be graded on a scale from 0 to 100. Reports are due one week after the completion of the experimental work. Two points will be deducted from the score for every day that the report is late. A lab report is required for each lab. The charts and tables in lab reports must be computer generated. The major part of most reports should be done using a computer spreadsheet program. Discussion and answers to questions may be handwritten if they are neat. Reports should include the following information. 1) Your name should be on each page. The first page should also include, the title and number of the experiment, the name(s) of your partner(s) and the dates on which the experiment was performed and submitted. 2) Tables ALL INFORMATION NEEDED TO REPRODUCE THE RESULTS OF THE EXPERIMENT MUST BE GIVEN IN THE REPORT. DATA IS USUSALLY BEST PRESEMTED IN A TABLE OR TABLES. The experimental uncertainties of the data should be included with each data entry. All tables should have titles. The units of all tabulated data should be listed in the table. 3) Plots 2 CHEM 322L INSTRUMENTAL ANALYSIS LAB SYLLABUS Dr. DiLella Spring 2011 All plots should be computer generated. Plots should be constructed so that they can be read to the maximum possible precision. Adjust the x and y ranges so that they are slightly larger than those needed to contain all of the data points. Do not squeeze the whole plot into a corner. The axes on plots should be labeled and the dimensions (units) of all quantities should be indicated. When a line is appropriate, use the computer to calculate and draw the best line through the data points. The plot should include both the experimental points and the best line. A sample calculation showing every step used to treat the raw data should be presented. This part may be handwritten but must be neat. It is neither necessary nor desirable to show repeated examples of the same basic calculation. Describe the purpose of each step and indicate clearly the source of every quantity that you use in the calculation. Appropriate units should be used throughout the calculation. 4) Error analysis Make a quantitative estimate of the quality of the results. For every measurement include an experimental uncertainty. The best time to determine the uncertainties is while you are doing the experiment. For example, if you measure a 10 mL volume that you believe to be accurate to 0.01 mL you record the measurement as 10.00 ± 0.01 mL. Tolerances for volumetric glassware are tabulated in many texts. In many cases it will be up to you to estimate an appropriate uncertainty. The estimate will be based on the reproducibility of the data if more than one reading was made. In cases where only one reading is made you must make an educated guess which should, of course, include a justification. Remember that not all sources of error are equally important. You should ignore those sources that are insignificant but you should give a quantitative justification when you do so. After determining the confidence limits for the raw data a propagation of errors treatment is needed. (see references). Remember that the propagation of error treatment depends on the accurate determinations of the experimental uncertainty. Special methods are used to handle the uncertainty for quantities which are obtained from graphical data. The method of least squares is the preferred method. The least squares technique is discussed in references 1. 5) Be sure to discuss all results. The discussion does not have to be long in most cases but you should summarize the results and indicate confidence limits. Answer all questions indicated in the syllabus. References 1. J. R. Robinson, E. M. Skelly Frame, and G. M. Frame II, “Undergraduate Instrumental Analysis”, 6th ed., Marcel Dekker (2005) 2. Daniel C. Harris, "Quantitative Chemical Analysis", Seventh Edition W.H. Freeman and Company (2007). 3