Table S1 - Figshare
advertisement

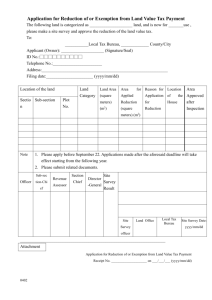
Table S1. Data-collection grid to extract information from each article Section Article reference Variable Name ID Information Unique index specific of the article Source From which study subset Authors Title Journal Full names and initials Title of the article Title of the journal Year Volume Publication year of the article Volume in which the article was published Issue in which the article was published The article’s first and last page numbers in the journal Date of reception of the first manuscript submission Number Number a_date Date of manuscript acceptance Date format: dd/mm/yyyy e_date Date of the online publication Date format: dd/mm/yyyy p_date Date of the print publication Date format: dd/mm/yyyy Issue Pages r_date Format Specification Incremental integer automatically generated by the database system Dropdown menu list: Hong Kong subset Toronto subset Combined subset Text Text Journal full or abbreviated name according to either the Medline or Web of Science format Table continues on the following page. 1 Text Text Date format: dd/mm/yyyy Comment e.g., 1, 2, 3, …, 311 e.g., Lee, T. S. Full title Medline format (e.g., Emerg Infect Dis); Web of Science format (e.g., Emerging Infectious Diseases) e.g., 352–58 Only entered when mentioned in the article or the Medline database Only entered when mentioned in the article or the Medline database Only entered when mentioned in the article or the Medline database Only entered when mentioned in the article or in the Medline database Table S1. (Continued) Section Classification of the study Variable Name Type Information Study type Domain Main research domain of the study Setting Study setting Design Format Specification Dropdown menu list: Descriptive epidemiology Analytic epidemiology Theoretical epidemiology Experimental epidemiology Dropdown menu list: Description of the outbreak Search for causative agent Transmission Risk factors Clinical presentations Diagnostic assays Treatments and medical interventions Prognosis Medical decision-making Prevention and control measures Psychobehavioral investigation Dropdown menu list: Hospital Community Both Dropdown menu list: Cross-sectional study Descriptive cohort study (longitudinal study) Prospective cohort study Historical cohort study Case–control study Intervention trial Clinical trial Mathematical modeling study Molecular study Diagnostic study Epidemiological study design Table continues on the following page. 2 Comment Hospital study refers to those conducted in any medical setting (e.g., hospital, clinic) Table S1. (Continued) Section Characteristics of the study population Variable Name Population Information Type of population Format Specification Check boxes: Definition Definition criteria of the SARS cases Location Population recruitment location Sample_size ss_init Sample size of the study Initial sample size of the study SARS patients General population Healthcare workers (non SARS) Othera Check boxes: WHO SARS-case definition CDC SARS-case definition Othera Not specified Check boxes: Hospital Place of work Place of residence Othera Not specified Number Number ss_fin Final sample size of the study Number data_coll Type of data collection Check boxes: Questionnaire Biological specimen collection Physical examination(s) Environmental sample Hospital, medical or exposure records Secondary data Othera Table continues on the following page. 3 Comment Only entered when applicable, depending on study design Only entered when applicable, depending on study design Secondary data refer to analyses of data that had been collected and used in a previous study. Table S1. (Continued) Section Characteristics of the study data Variable Name dc_start Information First date of data collection Format Specification Date format: dd/mm/yyyy dc_end Quality Last date of data collection Procedures used to guarantee the quality of data Description of the statistical or mathematical methods used in the study Software used for data management Software used for data analysis Date format: dd/mm/yyyy Text Analysis sft_dm sft_da aIn bIn Comment e.g., double data entry, data checking procedure(s) Yesb/not specified/not applicable Yesb/not specified Yesb/not specified/not applicable such a case, a dependent variable is entered (text format) to specify the meaning of “other”. such a case, a dependent variable is entered through an incremental group of checked boxes to indicate the method(s) or the software used. 4