eche 311 chemical thermodynamics
advertisement

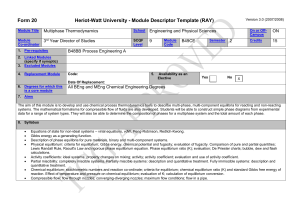
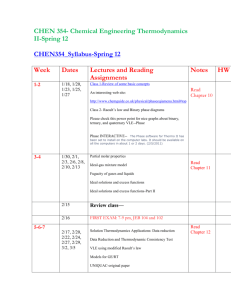
ECHE 311 CHEMICAL ENGINEERING THERMODYNAMICS Fall 2007, 11:00 AM. -12:15 PM TTH, SWGN 2A 21 Professor: Office: Tel: Email: Web site: Office Hrs: Dr. Branko N. Popov 2C19 777-7314 popov@engr.sc.edu http://www.che.sc.edu Monday 11-12 am, Fri. 11-12 am and by appointment at 777-7314 or by email. Teaching Assistants: Textbook: Smith, Van Ness, and Abbott, “Introduction to Chemical Engineering Thermodynamics” 7th ed., McGraw-Hill, 2005. Ref. Textbooks: Elliot and Lira, “Introduction to Applied Thermodynamics,” Prentice Hall PTR, 1999. K. S. Pitzer, “Thermodynamics,” 3rd Edition, Mc. Graw-Hill, 1995. Prerequisite: ENGR 290, Thermodynamic Fundamentals Course Objectives: The students will learn the concepts of chemical engineering systems, evaluation of thermodynamic property changes of pure materials, solution thermodynamics of singlephase multicomponent systems, phase and chemical and electrochemical reaction equilibrium, electrochemical equilibrium. Computer Usage: Extensive use of spreadsheets, MathCad or Maple and graphing packages. 1 Expected Knowledge: To pass this course the student must demonstrate that he/she knows how to set up and solve phase equilibrium problems recognize thermodynamic fundamentals and distinguish thermodynamic models(e.g. compressibility equation, Equations of State, activity coefficient models, etc). calculate vapor-liquid equilibrium (bubble point, dew point and flash). determine and locate-pure component thermochemical data. determine the Nernst potential , electrochemical equilibrium set up and solve problems in reaction equilibrium Grading: 2 exams 40% September 25, October 30 /2007 Weekly Quizzes 10% Homework 25%: Follow ChE Handbook Format* Final Exam 25%: Monday , Dec. 17 @ 9:00 AM Total 100% *Homework must be submitted on time. Late homework will not be accepted unless valid reasons exist. Exams (open book) will be related to the homework and lecture material. 2 ECHE 311 Approximate Schedule, Fall 2007 Final Exam Monday December 17th @ 9:00 AM Holidays September 3rd , Labor Day, October 11-12, (Fall Break), Nov 21-Nov 25, Thanksgiving Date Topic and Reading Comment Unit 1 First and Second Law/Equation of States/Residual Properties 8/23 Orientation. Review of Basic Concepts: Mass and Energy Balances: First day of class, Read SVNA Ch 1,2,4,, B .N .Popov “Handouts” 8/28 The Reversible Process, Enthalpy, Heat capacity: Read: SVNA Ch2 , B. N. Popov” Handouts “The Fist Law” 8/30 Second Law, Entropy, Read SVNA Ch. 5 thru Section 5.9, B.N. Popov Handouts ”The Second law of Thermodynamics,” 9/4 Properties of pure fluids: Equation of State-Virial Equation Read SVNA Ch.3, Problem Solving (P.S.) 9/6 Cubic Equations of State, Problem Solving 9/11 Generalized Correlations for Gases and Liquids, 9/13 Property Relations Homogeneous Phases, SVNA Section 6.1 6.2 9/18 Fundamental Equation: Homogeneous Phases, Residual Properties: Read SVNA Section 6.1 6.2, 6.3, (P. S). 9/20 Two phase systems / SVNA, Section 6.4, (P.S) Generalized Properties Correlation for Gases: SVNA, Section 6.7, (P.S). Review Unit 1. 9/25 EXAM 1-Unit 1 Unit 2 Solution Thermodynamics Theory: Phase Equilibrium 9/27 10/2 Criterion for Phase Equilibrium: Chemical Potential, Read SVNA Ch.11 Section 11.1, and 11.2. (P.S) Fugacity from an EOS-Pure Component, Read SVNA Section 11.5 3 10/4 Generalized Correlation for the Fugacity Coefficient (P.S) 10/9 Ideal Solution 10/11-12 10/16 Fall Break Solution Thermodynamics Liquid Phase Properties from VLE data: Read SVNA Ch12 10/18 Activity Coefficients from experimental data (P.S.) 10/23 Models for the Excess Gibbs Energy and Margules Equation/ (P. S.) 10/25 Review Unit 2 10/30 EXAM 2 on Unit 2 Unit 3 Gamma/Phi Method/Electrolyte Equilibrium/Reaction Equilibrium 11/1 Gamma-Phi method for VLE, Raoult’s law, Modified Raoult’s law, Read SVNA Ch. 10, B .N .Popov “Handouts” 11/6 Dew point and Bubble point calculations 11/8 Flash calculations: Problem Solving 11/13 Reaction Equilibrium , Read SVNA Chapter15, Section 13.1, 13.2, 13.3, 13.4 13.5, 13.6, 13.7 11/15 Application of equilibrium criteria to chemical reactions/ Standard Gibbs Free Energy change and the equilibrium constant (Effect of Temperature, Van’t Hoff Equation Gas-Phase Reactions 11/20 Application of equilibrium criteria to chemical reactions/ Standard Gibbs free energy change and the equilibrium constant (Effect of temperature, Van’t Hoff Equation gas-phase reactions 11/22 No classes 11/27 Application of equilibrium criteria to chemical reactions/ Standard Gibbs free energy change and the equilibrium constant (Effect of temperature, Van’t Hoff Equation gas-phase reactions 11/29 Liquid-phase reactions, Equilibrium conversion for single reactions 12/4 Review Unit 3 Thanksgiving 12/6 Last Day of Classes 4