ECHE 311 CHEMICAL THERMODYNAMICS
advertisement

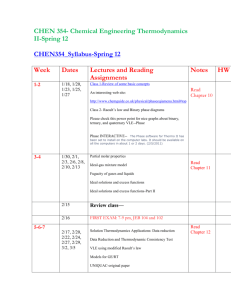
ECHE 311 CHEMICAL ENGINEERING THERMODYNAMICS SPRING 2011, 11:00 AM. -12:15 PM TTH, SWGN 2A 24 Professor: Office: Tel: Email: Dr. Branko N. Popov 2C19 SWG Eng. Center 803-777-7314 popov@cec.sc.edu http://www.che.sc.edu/faculty/popov/default.htm Web site: Office Hrs: Monday 11-12 am, Fri. 11-12 am and by appointment at 777-7314 or by email. Teaching Assistants: Tae kuen Kim Office: 2B33, SWG. Eng. Center Phone: 803-576-5634 Email: kim255@email.sc.edu Phone: Email: Won Suk Jung 2B33, SWG. Eng. Center 803-576-5634 jungw@email.sc.edu Phone: Email Xie Tianyuan 2B33, SWG. Eng. Center 803-576-5634 xie7@emial.sc.edu Textbook: Smith, Van Ness, and Abbott, “Introduction to Chemical Engineering Thermodynamics” 7th ed., McGraw-Hill, 2005. Ref. Textbooks: Elliot and Lira, “Introduction to Applied Thermodynamics,” Prentice Hall PTR, 1999. K. S. Pitzer, “Thermodynamics,” 3rd Edition, Mc. Graw-Hill, 1995. 1 Prerequisite: ENGR 290, Thermodynamic Fundamentals Course Objectives: The students will learn the concepts of chemical engineering systems, evaluation of thermodynamic property changes of pure materials, solution thermodynamics of single-phase multicomponent systems, phase and chemical and electrochemical reaction equilibrium, electrochemical equilibrium. Computer Usage: Extensive use of spreadsheets, MathCad or Maple and graphing packages. Expected Knowledge: To pass this course the student must demonstrate that he/she knows how to set up and solve phase equilibrium problems recognize thermodynamic fundamentals and distinguish thermodynamic models(e.g. compressibility equation, Equations of State, activity coefficient models, etc). calculate vapor-liquid equilibrium (bubble point, dew point and flash). determine and locate-pure component thermochemical data. determine the Nernst potential , electrochemical equilibrium set up and solve problems in reaction equilibrium Grading: 2 exams 50% February 10, March 24 /2011 Weekly Quizzes 5% Homework’s 15% Follow ChE Handbook Format* Final Exam 30% Tuesda, May 3 @ 9:00 AM Total 100% *Homework must be submitted on time. Late homework will not be accepted unless valid reasons exist. Exams (open book) will be related to the homework and lecture material. 2 ECHE 311 Approximate Schedule, SPRING 2011 Final Exam Holidays Date Tuesday May 3 @ 9:00 AM January 17th , Dr. Martin Luther King, Jr. Service Day, March 6-13 Sun-Sun, (Spring Break), Topic and Reading Comment Unit 1 First and Second Law/Equation of States/Residual Properties 1/11 Orientation. Review of Basic Concepts: Mass and Energy Balances: 1/13 First day of class, Read SVNA Ch 1,2,4,, B .N .Popov “Handouts” The Reversible Process, Enthalpy, Heat capacity: Read: SVNA Ch2 , B. N. Popov” Handouts “The Fist Law” 1/18 Second Law, Entropy, Read SVNA Ch. 5 thru Section 5.9, B.N. Popov Handouts ”The Second law of Thermodynamics,” 1/20 Properties of pure fluids: Equation of State-Virial Equation Read SVNA Ch.3, Problem Solving (P.S.) 1/25 Cubic Equations of State, Problem Solving 1/27 Generalized Correlations for Gases and Liquids, 2/1 Property Relations Homogeneous Phases, SVNA Section 6.1 6.2 2/3 Fundamental Equations: Homogeneous Phases, Residual Properties: Read SVNA Section 6.1 6.2, 6.3, (P. S). 2/8 2/10 Two phase systems / SVNA, Section 6.4, (P.S) Generalized Properties Correlation for Gases: SVNA, Section 6.7, (P.S). Review Unit 1. EXAM 1-Unit 1 Unit 2 Solution Thermodynamics Theory: Phase Equilibrium 2/15 Criterion for Phase Equilibrium: Chemical Potential, Read SVNA Ch.11 Section 11.1, and 11.2. (P.S) 3 2/17 Fugacity from an EOS-Pure Component, Read SVNA Section 11.5 2/22 Generalized Correlation for the Fugacity Coefficient (P.S) 2/24 Ideal Solutions 3/1 Solution Thermodynamics Liquid Phase Properties from VLE data: Read SVNA Ch12 3/3 Activity Coefficients from experimental data (P.S.) 3/6-3/13 Spring brake 3/15 Models for the Excess Gibbs Energy and Margules Equation/ (P. S.) 3/17 Review Unit 2 3/22 EXAM 2 on Unit 2 Unit 3 Gamma/Phi Method/Electrolyte Equilibrium/Reaction Equilibrium 3/24 Gamma-Phi method for VLE, Raoult’s law, Modified Raoult’s law, Read SVNA Ch. 10, B .N .Popov “Handouts” 3/29 Dew point and Bubble point calculations 3/31 Flash calculations: Problem Solving 4/5 Reaction Equilibrium , Read SVNA Chapter15, Section 13.1, 13.2, 13.3, 13.4 13.5, 13.6, 13.7 4/7 Application of equilibrium criteria to chemical reactions/ Standard Gibbs Free Energy change and the equilibrium constant (Effect of Temperature, Van’t Hoff Equation Gas-Phase Reactions 4/11 Application of equilibrium criteria to chemical reactions/ Standard Gibbs free energy change and the equilibrium constant (Effect of temperature, Van’t Hoff Equation gas-phase reactions 4/14 Application of equilibrium criteria to chemical reactions/ Standard Gibbs free energy change and the equilibrium constant (Effect of temperature, Van’t Hoff Equation gas-phase reactions 4/19 Application of equilibrium criteria to chemical reactions/ Standard Gibbs free energy change and the equilibrium constant (Effect of temperature, Van’t Hoff Equation gas-phase reactions 4/21 Liquid-phase reactions, Equilibrium conversion for single reactions -Review Unit 3 4/25 Last Day of Classes 4