Investigating Acids and Bases
advertisement

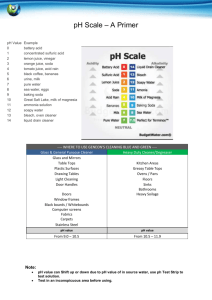
Lab # _______: Investigating Acids and Bases Date ________ Introduction Scientists use solutions called indicators to measure the acidity of a solution. An indicator solution changes color as the acidity (pH) changes. In this lab you will first investigate the use of three different indicators -red cabbage juice, litmus paper, and pH paper. (Litmus paper and pH paper are made by soaking paper in a solution that changes color as acidity changes.) You will then use these indicators to investigate a common acid base reaction. Procedure – Part A – Using Cabbage Juice Indicator 1. Place 10 drops of red cabbage juice in each of 8 wells in a spot plate. 2. Add 3 drops of dilute HCl to the first well. Mix gently. Record any changes in color. 3. Add 3 drops of dilute NaOH to the next well. Mix gently. Record any changes in color. 4. Add 3 drops of lemon juice to the next well. Mix gently. Record any changes in color. 5. Add 3 drops of ammonia to the next well. Mix gently. Record any changes in color. 6. Add 3 drops of vinegar to the next well. Mix gently. Record any changes in color. 7. Add 3 drops of dish detergent solution to the next well. Mix gently. Record any changes in color. 8. Add a small amount of baking soda to the next well. Mix gently. Record any changes in color. 9. Add a small amount of cream of tartar to the next well. Mix gently. Record any changes in color. Procedure – Part B – Using Litmus Paper and pH Paper 1. Put a drop of each of the test substances (the pure substances, NOT the cabbage juice mixtures!) on a separate piece of litmus paper. For the baking soda and cream of tartar you will need to dissolve a small amount in water first. Record the final color of the litmus paper for each substance. 2. Put a drop of each of the test substances (the pure substances, NOT the cabbage juice mixtures!) on a separate piece of pH paper. For the baking soda and cream of tartar you will need to dissolve a small amount in water first. Compare the color of the pH paper to the scale on the dispenser. Record the pH of each substance. Procedure – Part C – Observing Acid-Base Reactions 1. (This part will be done as a demonstration) Put 3 ml of red cabbage juice into each of 2 test tubes. Measure the pH in the first tube with the pH probe. Now, while recording the pH with the probe gently blow through a straw into this test tube for 1 - 2 minutes, bubbling your exhaled breath into the cabbage juice. Compare the color in this tube to the color of the tube that wasn’t blown into. Record any differences in color and in pH. Repeat this procedure for the second tube without blowing and record the results. 2. Put a small amount of baking soda into each of 7 wells in the spot plate. Add 5 drops dilute HCl to the first tube. Record any changes. Repeat for the other 6 tubes, using NaOH, lemon juice, ammonia, vinegar, dish detergent, and cream of tartar solution (a small amount of cream of tartar dissolved in water). Record any changes you observe. 3. Dissolve a small amount of double acting baking powder in about 50 ml. of water. Observe carefully and record any changes. When you no longer see any reaction, heat the solution on a hot plate for a few minutes, watching carefully. Record any changes you observe. Questions (Questions with an * at the end must be answered in complete sentences.) 1. Which of the indicators you used gave the most precise measure of acidity? 2. What pH ranges are considered acidic? 3. Which of the substances you tested are acids? 4. What color did acidic solutions turn the cabbage juice? 5. What color did the acidic solutions turn the litmus paper? 6. What pH ranges are considered basic? 7. Which of the substances you tested are bases? 8. What color did basic solutions turn the cabbage juice? 9. What color did the basic solutions turn the litmus paper? 10. What substance are you adding to the cabbage juice when you bubble your exhaled breath through it? 11. What changes in color and pH occurred when you blew into the water? 12. What can you infer about what happens to pH when you add CO2 to water?* 13. When you add CO2 to water the following chemical reaction occurs: H O CO H CO 2 2 2 3 What is the name of the product of this reaction? (Hint: Look at the list of common acids and bases on the Acids and Bases worksheet.) 14. How does the name of the product of this reaction help to explain why the pH changed when you blew your breath through the water?* 15. What substances caused a reaction when added to baking soda? 16. What did all these substances have in common?* 17. What can you infer about what will happen when you add any acid to baking soda?* 18. Based on your answers to questions 12 – 14 what gas do you think is made when an acid is mixed with baking soda? 19. A cake rises because gas bubbles form in the batter. Some recipes call for baking soda and cream of tartar to make a cake rise. Explain why this combination works and predict what gas is made.* 20. Based on your observations, why do you think double-acting baking powder is called “double-acting”?* Data Chart – Parts A & B Substance Color of Cabbage Juice Color of Litmus Paper pH (from pH paper) Dilute HCl Dilute NaOH Lemon juice Ammonia Vinegar Dish detergent solution Baking soda Cream of tartar Data Chart – Part C1. Tube #1 (blown in) Tube #2 (not blown in) Color at start pH at start Color at end pH at end Data Chart – Part C2 Substance Dilute HCl Dilute NaOH Lemon juice Reaction with baking soda Data – Part C3 Observations of double acting baking powder: Ammonia Vinegar Dish detergent solution Cream of tartar