Acids and Bases

Adan Hussain January 28, 2010
Acids and Bases
Objective: To introduce common acids and bases in the household and the pH scale.
Introduction: Acid-base reactions occur everywhere at all times such as when taking basic
Pepto Bismal in order to neutralize acid build up in our stomach or when buffers protect us from chemicals when they come in contact with our skin. In this activity, students will identify different acids and bases in their households to become familiar with the concept, and they will get a brief introduction the pH scale.
Materials:
Clorox (Bleach)
Liquid Soap (preferably clear)
Orange Juice
Snow or Water
Any clear soda
Lemon Juice
Vinegar
Baking Soda solution (baking soda mixed in water)
Test tubes or any clear containers to pour solutions in
Test tube or container tops
Pipettes or measuring cups
Cabbage Juice
Safety: Use of many chemicals.
Pre-Lab: Have Students do Pre-Lab activity
Procedure:
1.
Have eight test tubes ready for each group or student. You may pre-fill some of the test tubes with household solutions to save time. However, measuring should be an experience the students should get.
2.
Using measuring cups and/or pipettes, have the students fill 1/3 of their test tubes with household solutions (if they are not pre-filled already). Make sure they label or mark their test tubes so they do not mix them up.
3.
Using measuring cups and/or pipettes, have the students add drops of cabbage juice to each solution
4.
Close the tops of the test tubes and mix solution. Make sure the test tube is closed tightly.
1
Adan Hussain January 28, 2010
5.
Let them record the color they see on the color scale. Have students hold a white sheet of paper behind the test tube if they have trouble seeing the color.
6.
If there is no significant color change to a solution, open to the test tube, add more cabbage juice and mix again. Some solutions require more cabbage juice than others so they can add as much as they need.
7.
Answer Questions on Lab
Expected Results:
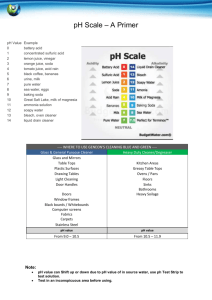
For the Clorox, baking soda and liquid soap, the cabbage juice should turn blue, green or any variation of those two colors. For the Vinegar, orange juice, lemon juice and soda, the cabbage juice should turn different shades of pink-red. The water or snow should stay purple, but if there is a color change, then that would be mean that our atmosphere is too polluted.
Supplementary Exercises:
Test other items in the classroom such as food from lunch or chalk
Mix different solutions and find out their pH
2
Adan Hussain January 28, 2010
Pre-Lab
Where do you think these items would fall on the color scale?
Bleach Soda
Baking Soda Lemon Juice
Orange Juice Vinegar
Water Liquid Soap
3
Adan Hussain January 28, 2010
Name_________________________
1.
Take Pipette and add 5 drops of cabbage juice to the first test tube.
2.
Cap the test tube and mix.
3.
Mark what color you see on this worksheet.
4.
Repeat for each item.
Orange Juice
Clorox
4
Adan Hussain
Baking Soda
Lemon Juice
Vinegar
Liquid Soap
January 28, 2010
5
Adan Hussain
Soda
January 28, 2010
Snow
What color do you think the cabbage juice would change to if you used apple juice? Why?
People take Pepto Bismal when there is too much acid in our stomach. What color do you think Pepto Bismal would have changed the cabbage juice to? Why?
What happens when you mix an acid and a base?
6
Adan Hussain January 28, 2010
Name some other acids in your home.
Name same other bases in your home.
What color was the cabbage juice when you added it to snow? What does that show? Why?
7