7.4 Natural and Artificial Transmutation
advertisement

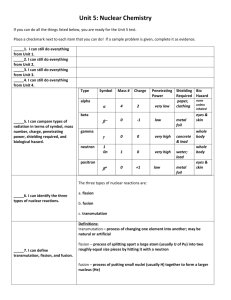
“Success is not final, failure is not fatal: it is the courage to continue that counts.” ~Winston Churchill Name: ________________________________ Date: ____________________________ 7.4 Natural and Artificial Transmutation AHS Instructor Ms. Kasia Rm 109 UNIT 7: Nuclear Chemistry 7.4 Natural and Artificial Transmutation AIM: DO NOW: 1. What subatomic particle is negatively charged? 2. Write the nuclear decay equation for gold-198. 3. If 1/8 of C-14 is left over, how many half-lives did the sample undergo? REVIEW: EXAMPLE: Write the spontaneous decay reaction for: 1. K‐42 2. Radon‐222 NATURAL AND ARTIFICIAL TRANSMUTATION • ____________________is a reaction in which we change one element to _________________ element. • There are two kinds of Transmutation reactions:_____________________ and ______________________ NATURAL TRANSMUTATION 1. The element undergoes spontaneous decay because it is unstable. 2. These reactions occur “spontaneously” (on its own) ARTIFICIAL TRANSMUTATION 1. For this change to happen, high‐energy particles are fired at the element. In this reaction, two things are being combined: “Success is not final, failure is not fatal: it is the courage to continue that counts.” ~Winston Churchill EXAMPLES EXAMPLES What do you notice about the examples? What do you notice about the examples? PRACTICE Identify whether the following reactions is a natural transmutation or artificial transmutation BALANCING NATURAL AND ARTIFICIAL TRANSMUTATION REACTIONS • Remember the law of conservation of mass! We must have a balanced equation! Check if the following Nuclear Equation is balanced. Directions: Balance the equation to determine what Balance Mass Numbers: Balance Atomic Numbers: So X is, ______ 9 +1 4 +1 = = 6 +____ 3+ ____ “Success is not final, failure is not fatal: it is the courage to continue that counts.” ~Winston Churchill Name: ________________________________ Date: ____________________________ 7.4 Natural and Artificial Transmuation AHS Instructor Ms. Kasia Rm 109 UNIT 7: Nuclear Chemistry 7.4 Natural and Artificial Transmutation Directions: Find what is missing Directions: Answer the following using your notes 1. What is the name of the process in which the nucleus of an atom of one element is changed into the nucleus of an atom of a different element? (1) decomposition (2) transmutation (3) substitution (4) reduction 2. Which type of reaction converts one element to another element? (1) neutralization (2) polymerization (3) substitution (4) transmutation 3. Which nuclear equation represents a natural transmutation? 4. Given the nuclear equation: “Success is not final, failure is not fatal: it is the courage to continue that counts.” ~Winston Churchill 5. Which reaction is an example of natural transmutation? 6. Radioactive cobalt‐60 is used in radiation therapy treatment. Cobalt‐60 undergoes beta decay. This type of nuclear reaction is called (1) natural transmutation (2) artificial transmutation (3) nuclear fusion (4) nuclear fission 7. Types of nuclear reactions include fission, fusion, and (1) single replacement (2) neutralization (3) oxidation‐reduction (4) transmutation 8. Which equation is an example of artificial transmutation? 9. Given the nuclear equation: Which particle is represented by X? 10. The change that is undergone by an atom of an element made radioactive by bombardment with high‐energy protons is called (1) natural transmutation (2) artificial transmutation (3) natural decay (4) radioactive decay 11. The spontaneous decay of an atom is called (1) ionization (2) crystallization (3) combustion (4) transmutation 12. Given the nuclear reaction: This is an example of: (1) fission (3) artificial transmutation 13. Given the nuclear equation: Which particle is represented by X? (1) alpha (2) beta (3) neutron (4) positron (2) fusion (4) natural transmutation