Nuclear Chem Practice #1
advertisement

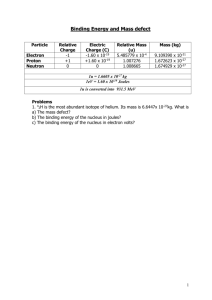
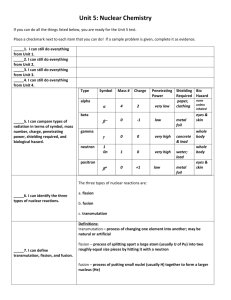
Name_______________________________________________ Regents Chemistry Date_________ Nuclear Chemistry Notes and Practice #1 Major Understandings 1. An “Artificial Transmutation” can be induced by the bombardment of a stable nucleus with high energy particles, causing the formation of a different element. 2. A “Particle Accelerator” can be used to induce an artificial transmutation reaction. It can only be used to accelerate charged particles. 3. A “Fission” reaction occurs when a large nucleus of one element is split into 2 smaller nuclei of different elements. 4. A “Breeder Reactor” is a special type of fission reactor that produces energy and more fuel at the same time. 5. A “Fusion” reaction occurs when 2 small nuclei are joined to form 1 larger nucleus of a different element. 6. “ E = mc2 “ can be used to explain how fission and fusion reactions produce energy. A small amount of matter (mass) is converted into a HUGE amount of energy. Skill #1 – Determining if a particle can be accelerated Key Ideas 1. Only charged particles can be accelerated. 2. Use Table O to look up the charge on a particle. 1. Complete the chart below for each of the following nuclear emanations: Name beta particle proton gamma radiation alpha particle positron neutron Notation Symbol Charge Can this emanation be accelerated by a particle accelerator? Skill #2 – Classifying transmutation reactions Key Ideas 1. All natural transmutation reactions will have only 1 reactant. The 1 reactant will decay by itself spontaneously. 2. All artificial transmutation reactions will have 2 or more reactants. The reactants will NOT react spontaneously (on earth). 3. A fission reaction is an artificial transmutation involving uranium – 235, uranium – 233, or plutonium – 239, and a neutron as the 2 reactants. 4. A fusion reaction is an artificial transmutation (on earth) involving hydrogen isotopes as the 2 reactants. Helium will be the product or one of the products. 2. Complete the chart below for each of the following transmutation reactions: Transmutation Reaction 238 U 4He + 234Th 2 27 235 U + 1n Kr + 140Ba + 3 1n C 14 N + -1e U + 1n 2 –1e + 222 n + P + 1n H + 2H 4He + 1n 14 238 93 30 Li + 1n 3H + 4He 3 1 H + 2H 4He Al + 4He 6 Rn Pu 239 131 14 Is this a natural or artificial transmutation? I 239 Pu 218 Po + 4He 90 Sr + -1e + 147 Ba + 3 1n 131 Xe N + 4He 1H + 17 O Is this an example of fission or fusion? Key Ideas Used