Emulsions: Types, Detection, & Pharmaceutical Applications
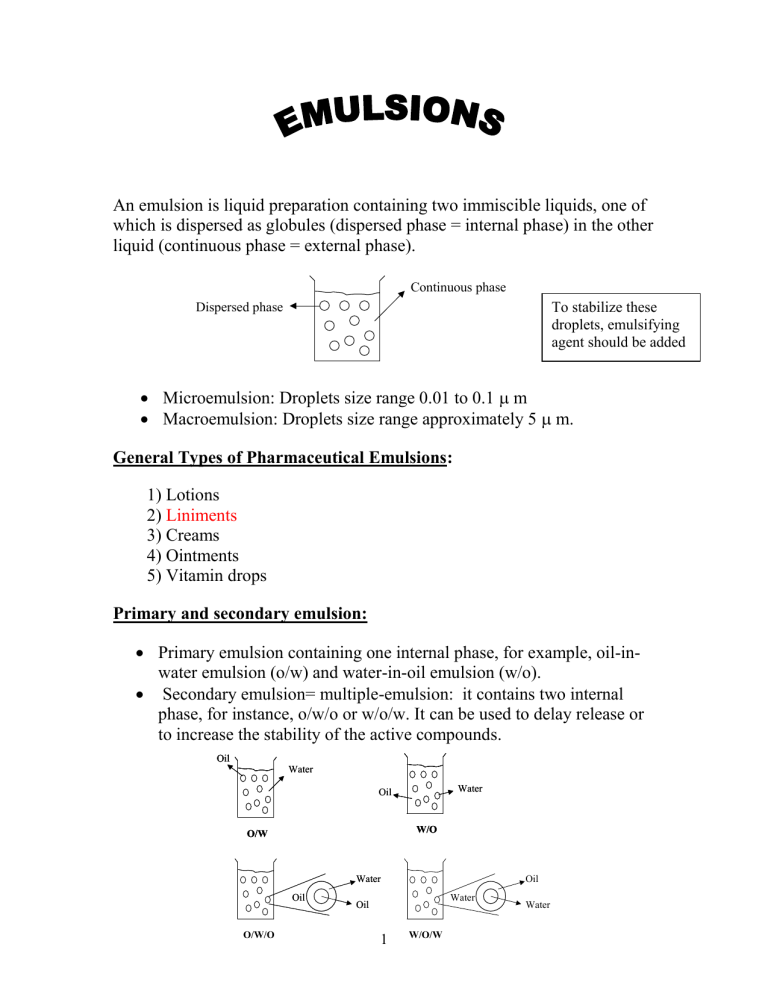
An emulsion is liquid preparation containing two immiscible liquids, one of which is dispersed as globules (dispersed phase = internal phase) in the other liquid (continuous phase = external phase).
Continuous phase
Dispersed phase To stabilize these droplets, emulsifying agent should be added
Microemulsion: Droplets size range 0.01 to 0.1
m
Macroemulsion: Droplets size range approximately 5
m.
General Types of Pharmaceutical Emulsions:
1) Lotions
2) Liniments
3) Creams
4) Ointments
5) Vitamin drops
Primary and secondary emulsion:
Primary emulsion containing one internal phase, for example, oil-inwater emulsion (o/w) and water-in-oil emulsion (w/o).
Secondary emulsion= multiple-emulsion: it contains two internal phase, for instance, o/w/o or w/o/w. It can be used to delay release or to increase the stability of the active compounds.
Water
Oil
Water
O/W/O
1
W/O/W
Emulsion Type and Means of Detection : using of naked eye, it is very difficult to differentiate between o/w or w/o emulsions. Thus, the four following methods have been used to identify the type if emulsions.
1) Dilution Test: based on the solubility of external phase of emulsion.
- o/w emulsion can be diluted with water.
- w/o emulsion can be diluted with oil.
Few drops of water
Water distribute uniformly O/W emulsion
Few drops of emulsion
Water separate out as layer
W/O emulsion
2) Conductivity Test: water is good conductor of electricity whereas oil is non-conductor. Therefore, continuous phase of water runs electricity more than continuous phase of oil.
Bulb
Electrode
= Bulb glows with O/W
= Bulb doesn’t glow with W/O
Emulsion
3) Dye-Solubility Test:
- Water-soluble dye will dissolve in the aqueous phase.
- Oil-soluble dye will dissolve in the oil phase.
2
What is look like under the microscope after mixing with suitable dye
Oil-soluble dye (e.g. Scarlet) Water-soluble dye (e.g. Amaranth dye)
W/O O/W O/W
W/O
4-Fluorescence test: oils give fluorescence under UV light, while water doesn’t. Therefore, O/W emulsion shows spotty pattern while W/O emulsion fluoresces.
Pharmaceutical applications of emulsions:
1.
To mask the taste
2.
O/W is convenient means of orally administration of water-insoluble liquids
3.
O/W emulsion facilitates the absorption of water-insoluble compounds comparing to their oily solution preparations (e.g. vitamins)
4.
Oil-soluble drugs can be given parentrally in form of oil-in water emulsion. (e.g Taxol)
5.
Emulsion can be used for external application in cosmetic and therapeutic uses.
3
Theories of Emulsification:
Incase of two immiscible liquids
An explanation of this phenomenon is because of cohesive force between the molecules of each separate liquid exceeds adhesive force between two liquids. This is manifested as interfacial energy or tension at boundary between the liquids.
Surface area
Interfacial tension
System is thermodynamically unstable “ high energy”
Therefore, to prevent the coalescence and separation, emulsifying agents have been used.
Types of emulsifying agents:
1.
Surface active agent: adsorbed at oil/water interface to form monomolecular film to reduce the interfacial tension
2.
Hydrophilic colloids: forming a multimolecular film around the dispersed droplet
3.
Finely divided solid particles: they are adsorbed at the interface between two immiscible liquid phases to form particulate film
4
A-Monomolecular adsorption
W=
. A
Surface free energy
Interfacial tension
Surface area
In emulsion, the surface area is high to maintain the dispersion of the droplets. Thus, based on the above equation surface free energy becomes high consequently. The only way to keep it low is to reduce the interfacial tension.
Surface active agent (SAA) is molecule which have two parts, one is hydrophilic and the other is hydrophobic. Upon the addition of SAA, they tend to form monolayer film at the oil/water interface.
Hydrophilic head
Water
Hydrophobic tail
Oil
The functions of surface active agents to provide stability to dispersed droplets are as following:
Form monomolecular film i.
Reduction of the interfacial tension ii.
Form coherent monolayer to prevent the coalescence of two droplet when they approach each other iii.
Provide surface charge which cause repulsion between adjust particles
Combination of surface-active agents is used most frequently. The combination should form film that closely packed and condensed
5
This figures shows schematic of oil droplets in an oilwater emulsion. You can see the orientation of a
Tween and a Span molecule at the interface
Figure (a) shows good combination, which forms excellent emulsion.
Figure (b) and (c) show poor emulsion due to lack of closely packed or lack of complexation, respectively.
6
Classification of surface-active agents:
Note that:
Anionic SAA are mainly used for external used.
Cationic SAA are used for external used. They have, also, good antimicrobial activity (e.g. Benzalkonium chloride)
Nonionic SAA are stable over wide range of pH. They are not affected by change in pH or addition of electrolytes. They are less toxic and main function to provide steric repulsion
7
B-Multimolecular adsorption
Polysaccharides
Acacia
Agar
Alginic acid
Carrageenan
Guar gum
Karraya gum
Tragacanth
Amphoterics
Gelatin
Synthetic or semi-synthetic polymers
Carbomer resins
Cellulose ethers
Carboxymethyl chitin
PEG-n (ethylene oxide polymer)
Hydrophilic colloids form multimolecular adsorption at the oil/ water interface. They have low effect on the surface tension.
Their main function as emulsion stabilizers is by making coherent multi-molecular film. This film is strong and resists the coalescence.
They have, also, an auxiliary effect by increasing the viscosity of dispersion medium.
Most of the hydrophilic colloids form oil-in-water emulsions.
Some of them can provide electrostatic repulsion like acacia, which contains Arabic acid and proteins (COOH and NH
3
)
C-Solid particle adsorption
Finely divided solid particles are adsorbed at the surface of emulsion droplet to stabilize them. Those particles are wetted by both oil and water (but not dissolved) and the concentration of these particles form a particulate film that prevent the coalescence.
8
Particles that are wetted preferentially by water from o/w emulsion, whereas those wetted more by oil form w/o emulsion
Note that they are very rare to use and can affect rheology of the final product
Size of the particle is very important, larger particles can lead to coalescence
Finely divided solids
Bentonite
Hectorite
Kaolin
Magnesium aluminum silicate
Montmorillonite
Aluminum hydroxide
Magesium hydroxide
Silica
Other emulsifying agents
Natural emulsifying agents:
1.
Egg yolk: it contains phospholipids and cholesterol. The main withdraw back is that spoils quickly; therefore, it can’t be used in industry. It is used for extemporaneous preparation.
2.
Wool fat: anhydrous lanolin, it is used to prepare w/o emulsion for external uses.
3.
Starch: it forms starch mucilage and it is restricted for enemas preparation.
9
4.
Cholesterol: it has stabilizing action; therefore, another emulsifier should be included.
How to control emulsion type during formulation? a.
Volume of internal and external phases controls the type of emulsion.
The smaller volume will be for the internal phase and the larger volume will be for external phase. In some cases, internal phases can be more than 50% of the total volume (see the following section) b.
Dominance of polar and non-polar characteristic of emulsifying agents (relative solubility of emulsifying agent in water and oil).
Dominance of polar part results in formation of o/w emulsion and dominance of non-polar part results in formation of w/o emulsion.
Note that polar groups are better barriers than non-polar; therefore, o/w emulsion can be prepared with more than 50 % of oil phase “ internal phase”.
What the factors that affect the choice of emulsion type?
The choice of emulsion depends on (1)-properties and uses of final products
(2)- the other material required to be present.
Oil-soluble drug is prepared in o/w emulsion due its solubility and its taste can be masked by adding flavoring agents
For intravenous injection “ i.v.” o/w emulsion is the only type could be used.
For intramuscular injection “i.m.” both o/w and w/o types of emulsion could be used. Water-soluble drug can be prepared in w/o emulsion to get prolonged action (depot therapy)
Topical application: o Semisolid emulsions are called creams and lotions
Oil in water emulsion
For insoluble drug
For local effect
Easily to wash from skin
Doesn’t have greasy texture of oily preparation
Acceptable by consumer
Water in oil emulsion
For water soluble drug
Can be use to hydrate the upper layer of stratum corneum (moisturizing cream)
Can increase the absorption of drug from these formulation
Can be used to clean skin from dirt
Not acceptable by consumer
10
Methods for preparation of emulsion:
In small scales such as in pharmacy-hospital labs, mortar and pestle are the needed equipments.
In large scale such as in pharmaceutical industry, different machines are used:
1.
2.
Mixer or mechanical stirring: the emulsion is prepared by agitation of emulsion ingredient
Colloid mills
3.
Emulsifying Agents:
1) Carbohydrate Materials:
Acacia, Tragacanth, Agar, Pectin. o/w emulsion.
2) Protein Substances:
Gelatin, Egg yolk, Caesin o/w emulsion.
3) High Molecular Weight Alcohols:
Stearyl Alcohol, Cetyl Alcohol, Glyceryl Mono stearate-----------o/w emulsion. cholesterol------------------------------------------------------- w/o emulsion
4) Wetting Agents:
Anionic, Cationic, Nonionic
11
o/w emulsion w/o emulsion
5) Finely divided solids:
Bentonite, Magnesium Hydroxide, Aluminum Hydroxide o/w emulsion.
Phase Inversion:
The relative volume of internal and external phases of an emulsion is important.
(increase) internal concentration (increase) viscosity up to a certain point.
Viscosity will decrease after that point.
At this point the emulsion has undergone inversion i.e. it has changed from an o/w to a w/o, or vice versa. In practice, emulsions may be prepared without inversion with as much as about 75% of the vol. of the product being internal phase.
Methods of Preparation of Emulsions:
1) Continental or Dry Gum Method:
"4:2:1" Method
4 parts (volumes) of oil [Ansel. 7th ed. page 369]
2 parts of water
1 part of gum
Acacia or other o/w emulsifier is triturated with oil in a perfectly dry
Wedgwood or porcelain mortar until thoroughly mixed. Glass mortar has too smooth a surface to produce the proper size reduction of the internal phase
(Do not use glass mortar). After the oil and gum have been mixed, the two parts of water are then added all at once and the mixture is triturated immediately.
2) English or wet Gum Method:
12
Same proportion of oil, water and gum are used as in the continental or dry gum method but the order of mixing is different. Mucilage of the gum is prepared by triturating acacia (or other emulsifier) with water. The oil is then added slowly in portions, and the mixture is triturated to emulsify the oil.
Should the mixture become too thick during the process, additional water may be blended into the mixture before another successive portion of oil is added.
3) Bottle or Forbes Bottle Method:
Useful for-
Extemporaneous preparation of emulsion from volatile oils or oleaginous substance of low viscosity. put powdered acacia in a dry bottle
Add 2 parts of oil
Thoroughly shake the mixture in the capped bottle. A volume of water approximately equal to the oil is then added in portions, the mixture being thoroughly shaken after each addition.
This method is not suitable for viscous oils (i.e. high viscosity oil).
Stability of Emulsion:
An emulsion is considered to be physically unstable if : a) The internal phase tends to form aggregates of globules. b) Large globules or aggregates of globules rise to the top or fall to the bottom of the emulsion to form a concentrated layer of the internal phase. c) If all or part of the liquid of the internal phase becomes "unemulsified on the top or bottom of the emulsion.
Separation of the internal phase from the external phase is called
BREAKING of the emulsion. This is irreversible.
-Protect emulsions against the extremes of cold and heat.
13
-Emulsions may be adversely affected by microbial contamination.
Gels and Magmas:
Gels are defined as semisolid systems consisting of dispersions made up of either small inorganic particles or large organic molecules enclosing or interpenetrated by a liquid. Magmas or Milks are gels consisted of floccules of small distinct particles. Gels and Magmas are considered colloids because they contain particles within the range of colloidal dispersions.
Examples of Magmas & Gels:
Bentonite Magma, NF:
Preparation of 5% bentonite, a native, colloidal hydrated aluminum silicate, in purified water.
Aluminum Hydroxide Gel, USP:
This is an aqueous suspension of a gelatinous precipitate composed of insoluble aluminum hydroxide and hydrated aluminum oxide, equivalent to about 4% of aluminum oxide.
Milk of Magnesia, USP:
This is a preparation containing between 7 and 8.5% of Magnesium hydroxide.
14






