6.3 - WHO archives
advertisement
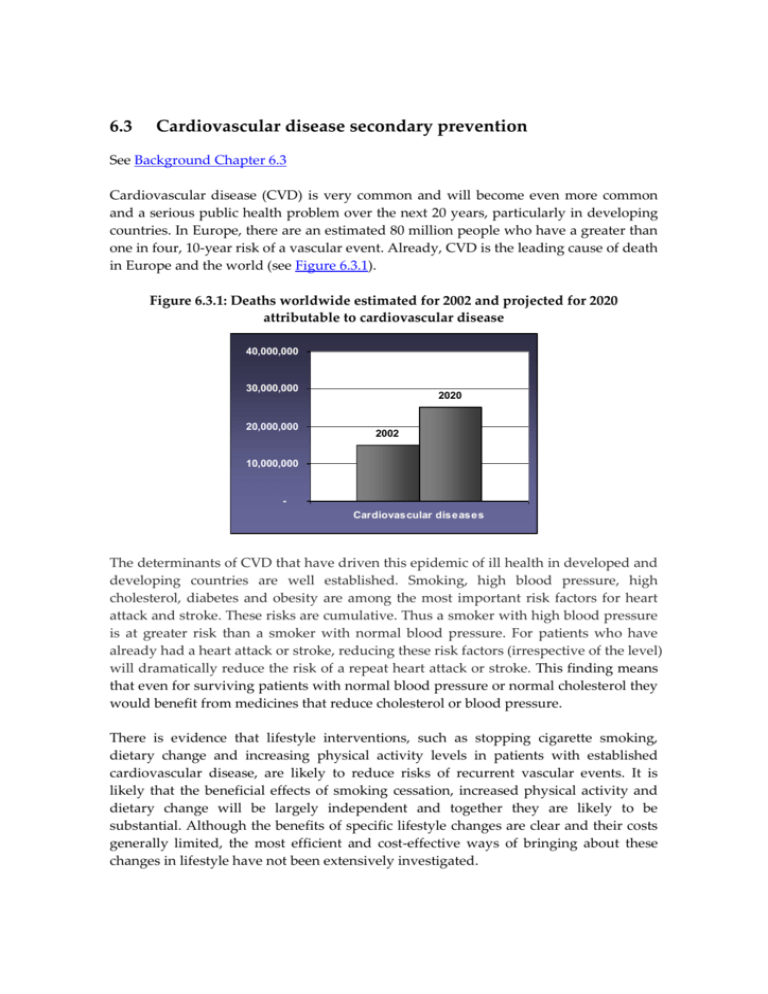
6.3 Cardiovascular disease secondary prevention See Background Chapter 6.3 Cardiovascular disease (CVD) is very common and will become even more common and a serious public health problem over the next 20 years, particularly in developing countries. In Europe, there are an estimated 80 million people who have a greater than one in four, 10-year risk of a vascular event. Already, CVD is the leading cause of death in Europe and the world (see Figure 6.3.1). Figure 6.3.1: Deaths worldwide estimated for 2002 and projected for 2020 attributable to cardiovascular disease 40,000,000 30,000,000 20,000,000 2020 2002 10,000,000 Cardiovascular diseases The determinants of CVD that have driven this epidemic of ill health in developed and developing countries are well established. Smoking, high blood pressure, high cholesterol, diabetes and obesity are among the most important risk factors for heart attack and stroke. These risks are cumulative. Thus a smoker with high blood pressure is at greater risk than a smoker with normal blood pressure. For patients who have already had a heart attack or stroke, reducing these risk factors (irrespective of the level) will dramatically reduce the risk of a repeat heart attack or stroke. This finding means that even for surviving patients with normal blood pressure or normal cholesterol they would benefit from medicines that reduce cholesterol or blood pressure. There is evidence that lifestyle interventions, such as stopping cigarette smoking, dietary change and increasing physical activity levels in patients with established cardiovascular disease, are likely to reduce risks of recurrent vascular events. It is likely that the beneficial effects of smoking cessation, increased physical activity and dietary change will be largely independent and together they are likely to be substantial. Although the benefits of specific lifestyle changes are clear and their costs generally limited, the most efficient and cost-effective ways of bringing about these changes in lifestyle have not been extensively investigated. With regard to smoking cessation, advice from health professionals has been shown to be effective. Although nicotine replacement therapy and antidepressant drugs have short-term benefits in smoking cessation their long-term Suggested formulation for secondary prevention of effectiveness is uncertain. heart attack It has been clearly Low-dose antiplatelet therapy (for example demonstrated that antiplatelet aspirin 75mg) therapy (with a medicine Full dose of a standard statin (for example like aspirin), 1 blood pressure simvastatin 40mg) lowering medicines (ACE Full dose of an angiotensin converting enzyme inhibitors or a diuretic) 2 , 3 and inhibitor (for example lisinopril 10mg), and Half dose of a beta-blocker (for example atenolol cholesterol lowering drugs 25mg) (statins),4 used separately or in combination, reduce the risk of Suggested formulation for secondary prevention of recurrent vascular events. stroke WHO has published two recent Low-dose antiplatelet therapy (for example reviews which strongly support aspirin 75mg) the use of these medicines for Full dose of a standard statin (for example secondary prevention (see simvastatin 40mg) Appendices 6.3). Full dose of an angiotensin converting enzyme inhibitor (for example lisinopril 10mg), and Half dose of a diuretic (for example hydrochlorothiazide 12.5mg) However, many patients with existing CVD receive substantially incomplete preventive therapy. The problem is that, for a variety of reasons, these patients are not prescribed the four different medicines they need to take. In 1999-2000, a major survey involving about 50 leading European hospitals identified major shortfalls in the secondary preventive care provided to patients who had been admitted with ischaemic heart disease.5 Ideally, each of the treatments should have been used in all patients but well under half of all patients were receiving all the recommended treatments. Another feature of the data was that there was only very limited improvement in treatment in comparison with a comparable survey completed a few years earlier. This information reinforces the need for novel strategies that will give physicians new ways of bridging the large gaps between defined optimal care and actual clinical practice. The simple solution to this deficiency is to develop and test a fixed-dose combination (FDC) product of these proven effective medicines. The research agenda proposed in this section is different to that of the other sections because this approach offers the greatest potential short- to-medium term impact of all of the possible research activities in this Report. The evidence for the use of FDCs to improve adherence, reduce costs, improve access and equity, and reduce medication errors for TB is convincing. However, to improve the treatment of CVD, there is a need for the testing of new formulations of two different, four-drug FDCs for the secondary prevention of heart attack and stroke (see text box on the previous page). Once such products are formulated there will be a need for clinical trials to assess the effect of these products on blood pressure, cholesterol and platelet functioning, adherence to guidelines, and the overall effect on mortality and morbidity. These trials should be undertaken in both developed and developing countries. While the EU Framework Programmes have not supported many clinical trials, trials for these proposed FDCs should be an exception. Because the component medicines are now off-patent, there is no incentive for innovative pharmaceutical companies to undertake such trials, and the generic companies do not generally have the capacity to do so. It is estimated that these four-drug FDCs could reduce the risk of a second heart attack or stroke by about two-thirds.6 If this combination proved effective, and cheap generic versions of the individual medicines were used, this would be a very cost-effective intervention. On the basis of the background paper, it is strongly recommended that a research agenda should be established to produce and test FDC products for secondary prevention of heart attack and stroke to improve adherence and prevent mortality and morbidity. 1 Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction and stroke in high risk patients. BMJ 2002;324(7329):71-86. 2 Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355(9200):253-259. 3 PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood pressure lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358(9287):1033-1041. 4 Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo-controlled trial. Lancet 2002;360(9326):7-22. 5 Wood D, EUROASPIRE I and II Group. Clinical reality of coronary prevention guidelines: a comparison of EUROASPIRE I and II in nine countries. Lancet 2001;357(9261):996-1001. 6 Wald N, Law M. A strategy to reduce cardiovascular disease by more than 80%. BMJ 2003;326(7404):1419-1425.