CHAPTER SUMMARY
advertisement

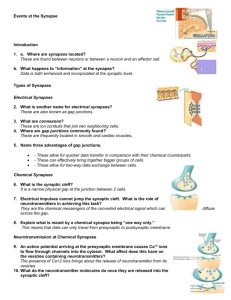
CHAPTER 4 SUMMARY Introduction Nerve and muscle cells are excitable because they can rapidly alter their membrane permeabilities and thus undergo transient membrane potential changes when excited. Compared to resting potential, a membrane becomes depolarized when its potential is reduced and hyperpolarized when its potential is increased. Changes in potential are brought about by triggering events that alter membrane permeability, in turn leading to changes in ion movement across the membrane. There are two kinds of potential change: (1) graded potentials, which serve as shortdistance signals that quickly die off within a close range of the small patch of membrane where they are first triggered, and (2) action potentials, the long-distance signals. Graded Potentials Graded potentials occur in a small specialized region of an excitable cell membrane. The magnitude of a graded potential varies directly with the magnitude of the triggering event. Graded potentials passively spread decrementally by local current flow and die out over a short distance. Action Potentials During an action potential, depolarization of the membrane to threshold potential triggers sequential changes in permeability caused by conformational changes in voltagegated channels. These permeability changes bring about a brief reversal of membrane potential, with Na+ influx being responsible for the rising phase (from -70 mV to -30 mV), followed by K+ efflux during the falling phase (from peak back to resting potential). Before an action potential returns to resting, it regenerates an identical new action potential in the area next to it by means of current flow that brings the previously inactive area to threshold. This self-perpetuating cycle continues until the action potential has spread throughout the cell membrane in undiminished fashion. There are two types of action potential propagation: (1) contiguous conduction in unmyelinated fibers, in which the action potential spreads along every portion of the membrane; and (2) the more rapid saltatory conduction in myelinated fibers, where the impulse jumps over the sections of the fiber covered with insulating myelin. The Na+–K+ pump gradually restores the ions that moved during propagation of the action potential to their original location to maintain the concentration gradients. It is impossible to restimulate the portion of the membrane where the impulse has just passed until it has recovered from its refractory period. The refractory period ensures the unidirectional propagation of action potentials away from the original site of activation. Action potentials occur either maximally in response to stimulation or not at all. Variable strengths of stimuli are coded by varying the frequency of action potentials, not their magnitude. Electrical and Chemical Synapses •Electrical synapses conduct action potentials from one cell to another via gap junctions. These are important for very fast reactions such as escape maneuvers. The more common means by which one neuron directly interacts with a target cell is through a chemical synapse. An action potential in the presynaptic neuron triggers an influx of Ca++ which triggers the release of a neurotransmitter, which combines with receptor sites on the postsynaptic (target) cell. •After diffusing away from the receptor, the neurotransmitter leaves the synapse, is taken up by the presynaptic cell, or is broken down by a local enzyme. •In a fast synapse, this combination alters the postsynaptic cell in one of two ways. The most typical response is a fast one involving the opening of chemical messenger-gated channels. If nonspecific cation channels that permit passage of both Na+ and K+ are opened, the resultant ionic fluxes cause an EPSP (due to the dominance of Na influx) , a small depolarization that brings the postsynaptic cell closer to threshold. On the other hand, the likelihood that the postsynaptic neuron will reach threshold is diminished when an IPSP, a small hyperpolarization, is produced as a result of the opening of either K+ or Cl- channels, or both. In an alternate, slow synaptic mechanism, an intracellular second messenger system, such as cyclic AMP, is activated by the neurotransmitter-receptor combination. Cyclic AMP can bring about channel opening or can have more prolonged effects within the cell, including alterations of the cell’s genetic expression. •Even though there are a number of different neurotransmitters, each synapse always releases the same transmitter to produce a given response when combined with a particular receptor. The response is terminated when the neurotransmitter is removed from the synaptic cleft by methods specific for the synapse. Many neurons co-secrete larger, more slowly acting neuropeptides along with the classical neurotransmitter. The neuropeptides largely function as neuromodulators at nonsynaptic sites on either the presynaptic or the postsynaptic neuron to enhance or suppress synaptic effectiveness. •At a neuromuscular synapse in vertebrates, acetylcholine (ACh) is always used, as a fast neurotransmitter; the enzyme acetylcholine esterase (AChE) breaks down ACh. Synapses and Neuronal Integration The interconnecting synaptic pathways between various neurons are incredibly complex due to convergence of neuronal input and divergence of its output. Usually, many presynaptic inputs converge upon a single neuron and jointly control its level of excitability. This same neuron, in turn, diverges to synapse with and influence the excitability of many other cells. Each neuron thus has the task of computing an output to numerous other cells from a complex set of inputs to itself. Depending on the combination of signals it is receiving from its various presynaptic inputs, at any given time a neuron may react by (1) transmitting action potentials along its axon, (2) remaining at rest and not passing any signals along, or (3) having its level of excitability increased or reduced. If the dominant activity is in its excitatory inputs, the postsynaptic cell is likely to be brought to threshold and have an action potential. This can be accomplished by either temporal summation (EPSPs from a single, repetitively firing presynaptic input occurring so close together in time that they add together) or spatial summation (adding of EPSPs occurring simultaneously from several different presynaptic inputs). Because the axon hillock has the lowest threshold, action potentials are initiated there. The frequency of action potentials reflects the magnitude of EPSP summation. If inhibitory inputs dominate, the postsynaptic potential will be brought farther than usual away from threshold. If excitatory and inhibitory activity to the postsynaptic neuron is balanced, the membrane will remain close to resting. Numerous external factors may alter neural signaling steps: some are deliberate pharmacological manipulations to achieve a desired result, some are accidents caused by poisons or disease processes, and some are toxins evolved in prey animals for defense and in predators for paralyzing or killing prey. Physical factors such as temperature and pressure also effect neural signaling.