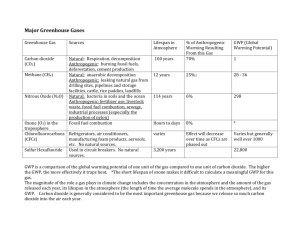
MOTM HFC-134a (1)
advertisement
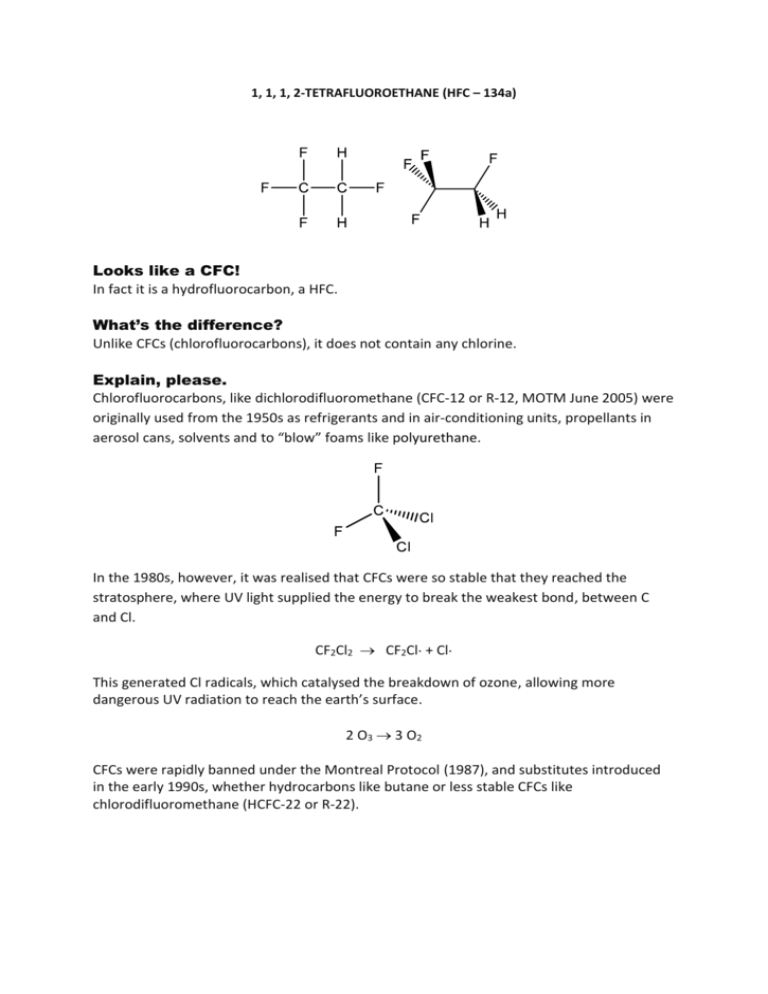
1, 1, 1, 2-TETRAFLUOROETHANE (HFC – 134a) Looks like a CFC! In fact it is a hydrofluorocarbon, a HFC. What’s the difference? Unlike CFCs (chlorofluorocarbons), it does not contain any chlorine. Explain, please. Chlorofluorocarbons, like dichlorodifluoromethane (CFC-12 or R-12, MOTM June 2005) were originally used from the 1950s as refrigerants and in air-conditioning units, propellants in aerosol cans, solvents and to “blow” foams like polyurethane. In the 1980s, however, it was realised that CFCs were so stable that they reached the stratosphere, where UV light supplied the energy to break the weakest bond, between C and Cl. CF2Cl2 CF2Cl· + Cl· This generated Cl radicals, which catalysed the breakdown of ozone, allowing more dangerous UV radiation to reach the earth’s surface. 2 O3 3 O2 CFCs were rapidly banned under the Montreal Protocol (1987), and substitutes introduced in the early 1990s, whether hydrocarbons like butane or less stable CFCs like chlorodifluoromethane (HCFC-22 or R-22). Later on, hydrofluorocarbons (HFCs) were introduced, which contain no chlorine and so do not destroy ozone. So that’s why HFC-134a is used? Yes, it has become the most widely-used HFC, not least because of its very low toxicity. It has a boiling point of -26 C, very similar to that of CF2Cl2, so it can be liquefied under pressure, making it well suited to refrigerant or air-conditioning systems. As it contains no chlorine, it cannot form ozone-depleting Cl· radicals, so it has zero ozone depletion potential. But it has a high global-warming potential, so it is not perfect either. What does that mean? Global warming potential (GWP) measures how much heat a “greenhouse gas” traps in the atmosphere, compared to the same mass of CO2 (which is given a GWP of 1). The GWP of tetrafluoroethane is 1430. Wow, so what are they doing about that? The European Union banned the use of HFC-134a in air-conditioners in new vehicle types from 1 January 2011 (with a view to a complete ban in all new cars from 1 January 2017) and in June 2013 China and the USA – the two biggest users - agreed to phase out HFCs. What alternatives are there to using 1,1,1, 2-Tetrafluoroethane? 2,3,3,3-tetrafluoropropene (HFO-1234-yf) is the preferred alternative at present. It has a much lower GWP (4) and much shorter lifetime in the atmosphere than HFC-134a, so is likely to be adopted by many auto manufacturers in the EU, though it is slightly flammable. Because of this problem, people are looking at using mixtures of HFO-1234-yf with HFC134a. Does 1,1,1,2-Tetrafluoroethane have any other uses? Being volatile and chemically inert, it is a useful solvent. It has been used to extract various organic molecules, like artemisinin from Artemisia annua, in place of flammable hydrocarbons and supercritical CO2. It is a propellant gas in aerosols, notably in drug delivery (though this can cause interference problems with infrared anaesthetic gas monitors), as well as in the duster gas used to clean computers. It has also been used by synthetic organic chemists in the synthesis of -fluoroenones. Bibliography R. P. Wayne, Chemistry of Atmospheres, 2nd ed., Oxford, Oxford University Press, 1991. O. V. Rattigan, D. M. Rowley, O. Wild, R. L. Jones and A. Cox, J. Chem. Soc. Faraday Trans., 1994, 90, 1819-1829. (mechanism of atmospheric oxidation of HFC-134a) J. Lu, H. Yang, S. Chen, L. Shi, J. Ren, H. Li and S. Peng, Catalysis Letters, 1996, 41, 221-224 (synthesis of HFC-134a) AR Brown and DW George, Pharm. Res., 1997, 14, 1542-1547 (HFC-134A driven aerosols) J. M. Bainbridge, S. Corr, M. Kanai and J. M. Percy, Tetrahedron Letters, 2000, 41, 971–974 (HFC-134a as a building block in syntheses of -fluoroenones) H. H. Emmen, E. M. G. Hoogendijk, W. A. A. Klöpping-Ketelaars, H. Muijser, E. Duistermaat, J. C. Ravensberg, D. J. Alexander, D. Borkhataria, G. M. Rusch and B. Schmit, Regul. Toxicol. Pharm., 2000, 32, 22–35 (human safety and pharmacokinetics of HFC 134a) E. A. Parson, Protecting the Ozone Layer, Oxford, Oxford University Press, 2003. S. Cagin and P. Dray, Between Earth and Sky, New York, Pantheon Books, 2003. P. M. de F. Forster, J.B. Burkholder, C. Clerbaux, P.F. Coheur, M. Dutta, L.K. Gohar, M.D. Hurley, G. Myhre, R.W. Portmann, K. P. Shine, T.J. Wallington and D. Wuebbles, J. Quant. Spectrosc. Radiat. Transfer, 2004, 93, 447-460 (radiative forcing of HFC-134a) P. D. Levin, D. Levin and A. Avidan, Br. J. Anaesth., 2004, 92, 865 - 869 (interference with infrared anaesthetic gas monitors) A. A. Lapkin, P. K. Plucinski and M. Cutler, J. Nat. Prod. 2006, 69, 1653-1664 (HFC 134a solvent in extraction of artemisinin) B. Barletta, P. Nissenson, S. Meinardi, D. Dabdub, F. S. Rowland, R. A. VanCuren, J. Pederson, G. S. Diskin and D. R. Blake, Atmos. Chem. Phys., 2011, 11, 2655–2669 (HFC-134a emission estimates) Y. Han, Q. Ma, L. Wang and C. Xue, J. Ocean Univ. China, 2012, 11, 562-568 (HFC 134a solvent in extraction of astaxanthin) V. Gochev, T.Girova, I. Stoilova, T. Atanasova, N. Nenov, V. Stanchev and A. Soyanova, J. BioSci. Biotech., 2012, 1, 135-139. (extractant of terpenes and terpenoids from Cardamom) Y. Lee, D.-G. Kang and D. Jung, Int. J. Refrig., 2013, 36, 1203-1207 (HFO1234yf/HFC134a mixtures as refrigerant)