Modelling Dual Reflux PSA Cycles Using A Transfer Function
advertisement

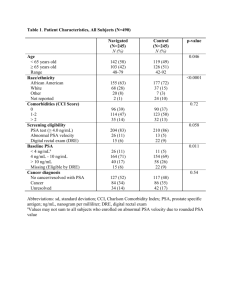
Modelling Dual Reflux PSA Cycles Using A Transfer Function Methodology for Separating Nitrogen and Methane in LNG Production Yechun Zhang1, Thomas Saleman2, Kevin Li2, Wei Yu1, Eric F. May2, Brent R. Young1* 1 The University of Auckland and 2The University of Western Australia *Email: b.young@auckland.ac.nz I. Abstract INTRODUCTION Liquefied natural gas (LNG) is an important energy source due to its clean combustion, low carbon emissions and high energy density. In 2011, 330.8 million cubic metres of LNG was traded around the world, which is a 10% increase compared to 2010 (300.6 million cubic metres) [1]. Global LNG supply is projected to grow 4.5% p.a. to 2030, more than twice as fast as total global gas production (2.1% p.a.) and faster than interregional pipeline trade (3.0% p.a.). LNG will contribute 25% of global supply growth in 2010-30, compared to 19% for 1990-2010 [2]. Liquefied natural gas (LNG) is an important energy source due to its clean combustion, low carbon emissions and high energy density. The methane-nitrogen separation process in LNG production is significant and challenging to ensure the nitrogen level in the LNG product is within specification, or to upgrade the vapour overheads from cryogenic distillation columns to meet specifications of vent/pipeline/fuel for gas turbine. Pressure swing adsorption (PSA) has been applied in the industry for separating gas mixtures. Its advanced configurations, such as stripping reflux PSA, enriching reflux PSA and Dual Reflux PSA (DR-PSA) have higher efficiencies which may lead to economically improved separation of methane and nitrogen. Literature has shown that DR-PSA cycles can in principle achieve perfect separation, and experimental demonstrations of DR-PSA with other mixtures have shown how it can concentrate dilute streams significantly even with low pressure ratios, and simultaneously achieve high product recovery rates. In this paper, commercial simulation software is used to simulate stripping reflux PSA and enriching reflux PSA according to Ebner and Ritter’s experiments in 2002 and 2010. A correlation based on the simulation results from both stripping reflux PSA and enriching reflux PSA is then developed using the transfer function method to simulate the cyclic steady state of Dual Reflux PSA. The results from the model were verified by comparing with Ebner and Ritter’s experimental data and showed a close match. The advantages and disadvantages of the modelling method are discussed. Western Australia contains a large quantity of natural gas, most of which is exported to the AsiaPacific region as LNG product. Australia is aiming to become one of the largest LNG exporting countries by 2020, and 140 billion Australian dollars will be invested in LNG plants in the next 10 years [3]. For example, as one of the largest natural gas reserves, Gorgon field will be producing 15 million tons of LNG per annum [4]. As some of these gas fields have a high nitrogen concentration, and the nitrogen specification in LNG product is limited to 1%, the design and operation of the methane-nitrogen separation process in LNG production is increasingly significant and challenging to ensure the nitrogen level in the LNG product is within specification [5]. Alternative application of methane-nitrogen separation will aim to upgrade the vapour overheads from cryogenic distillation columns to meet specifications of vent gas/pipeline gas or fuel for gas turbine. Pressure swing adsorption (PSA) has been applied in the industry for separating gas mixtures. However, due to the fact that current commercial adsorbents can only reach a methanenitrogen selectivity of around 3, conventional PSA columns are far from achieving efficient separations. For example, to decrease the methane concentration from 3% to 100ppm in a vent stream from a typical large scale LNG plant, a preliminary Keywords: Natural gas, LNG, Separation, Adsorption, Dual Reflux PSA 1 calculation revealed that 548 beds will be needed [3]. The methane concentration in the nitrogen enriched product could be decreased to 113ppm but the nitrogen recovery rate was only 7.4%. To increase the productivity and recovery rates, alternative configurations of PSA are needed to improve the overall separation efficiency. Dual reflux PSA (DR-PSA) is a unique PSA configuration which combines stripping PSA and enriching PSA. Theoretically DR-PSA has the potential to achieve perfect separation with high recovery rate, and the theory was supported by experiments in literature which have shown high separation efficiencies in binary separations [6-9]. This may lead to economically improved separation of methane and nitrogen. A typical layout of DRPSA unit is shown in Figure 1. However since equilibrium theory models assume perfect separation, it cannot be used to predict the product concentrations. Thus the motivation is present to develop other models that are capable of predicting product compositions. II. METHODOLOGY We have used three different methods to model DR-PSA units. First of all, the equilibrium theory model by Kearns and Webley was enhanced. The enhanced model is constructed on partial differential equations which describe the adsorption columns. The partial differential equations are reduced to ordinary differential equations by the method of characteristics, and are numerically and iteratively solved. The equilibrium theory model is able to find the optimized operating point for the DR-PSA unit given the total throughput and pressure ratio of the system. Some key optimized operating parameters such as enriching/stripping reflux ratios, feed step time and axial feed location can be obtained rather than specified. The system power consumption can also be calculated. A typical equilibrium model result in terms of the power consumption and total throughput is shown in Figure 2. Besides these, equilibrium theory model can be used to monitor the concentration profile inside the column for each step. The impact of the parameters and different configurations of DR-PSA on the concentration curve can be visually examined thus it helps us understanding DR-PSA processes. However due to the equilibrium theory model assuming perfect separation, it is overly simplified and cannot be used to predict product compositions. Figure 1. (a) DR-PSA is a combination of stripping PSA and enriching PSA with intermediate feed either at high pressure or at low pressure. (b) Pressure swing can either be done at the top or bottom. Total Throughput ()MMSCFD 7 The equilibrium theory model is the only model available for describing DR-PSA units at the moment. The first equilibrium theory model for DR-PSA was first published by Ebner and Ritter in 2004. The model assumed perfect separation of binary mixtures and proved the feasibility of sharp separations and high recoveries by DR-PSA using low pressure ratios. The effect of feed concentrations, pressure ratios, total throughput, reflux ratios and feed positions were discussed in detail [10]. Kearns and Webley significantly improved the equilibrium theory model in their two companion papers in 2006 by introducing the models for four different configurations, in terms of feed to low/high column and purge by lean/rich gas. The composition profile for each cycle was numerically calculated and the productivity and work consumption trade-off was discussed [11, 12]. 6 5 4 3 2 1 0 0 50000 100000 150000 200000 Energy Consumption (kW) Figure 2. A typical result obtained from equilibrium theory model: the relationship between total throughput and power consumption. To attempt to solve the above noted problem, Aspen Adsorption software was used to simulate the full cycle DR-PSA units. However we experienced difficulties when building the full 2 cycle model due an unstable and overly complicated pressure-flow network and internal material flow loops. To overcome this difficulty, here we propose an alternative method to estimate the efficiency of DR-PSA systems using the simulation results from the combination of a stripping PSA model and an enriching PSA model, which can be built in Aspen Adsorption. Both stripping and enriching PSA models in Aspen Adsorption can be solved much easier and quicker and thus can provide a faster prediction result and avoid building the full DR-PSA model. (a) Our model started with calculating the total material balance of the DR-PSA unit (regardless of each component), by defining the flow rate of each stream in Figure 3 (streams 1-7). This process mimics the actual material flow of a DR-PSA unit during adsorption/desorption when reaching cyclic steady state. The core idea of our model was to consider both the stripping PSA and enriching PSA of a DR-PSA unit as “transfer functions”, in terms of the relationships between their local feed composition and lean/rich product composition when reaching cyclic steady state. The transfer function is usually regressed from a certain amount of data points either from simulation models or experimental data. A typical example of transfer functions of stripping and enriching PSA are shown in Figure 4. (b) Figure 4. Examples of transfer functions of (a) a stripping PSA model and (b) an enriching PSA model, and their regressed curves. In our research work, Aspen Adsorption was adopted to build stripping/enriching PSA models and rigorously solve them to provide data points for regressing transfer functions. Then, the transfer functions of stripping/enriching PSA are combined according to the calculated total material balance for the DR-PSA unit to predict the product concentrations. An algorithm was developed in Matlab for the purpose of the iterative combination calculations. The detailed steps are illustrated in Figure 3 and as follows (Feed to HP column example): Step1: Set the flow rate at the local feed to the stripping PSA (stream 1) to desired value and make an initial assumption of its composition. Step 2: According to the regressed transfer function of the stripping PSA and the reflux ratio, calculate the flow rates and the compositions of lean product (stream 2) and waste (stream 3). Figure 3. Illustration of the algorithm steps in combining transfer functions of stripping/enriching PSA (Feed to high pressure configuration). Step 3: Transport the waste (stream 3) from stripping PSA part to the feed (stream 4) of enriching PSA part, then calculate the flow rates and compositions of rich product (stream 5) and waste (stream 6) according to the transfer function and reflux ratio of enriching PSA. 3 Step 4: Calculate the required flow rate of actual feed (stream 7) in order to mitigate the difference between stream 6 and stream 1. extended Langmuir 2 according to the equation/parameter definitions in Aspen Adsorption. Despite the fact that the dynamic material balance could not achieved due to adsorption/desorption processes, QLR, QLP, QHR and QHP were always guaranteed during simulation to ensure a stable productivity. Step 5: Since the composition of actual feed (stream 7) is given, the composition of stream 1 can be calculated and updated to start the next iteration. The simulation results and their comparison with experimental data are illustrated in Table 2. The result showed that the deviation was never greater than 0.1% for ethane concentration in lean product and never greater than 5.1% for ethane concentration in rich product. As the lean product and rich product recovery rates can be calculated by the lean/rich product split ratios and their compositions, they also exhibited a close match. The error in nitrogen recovery rate prediction was no more than 2% and the ethane recovery rate prediction error was no more than 6%. We believe that the main reasons for the deviations were because of the semi-isothermal assumption for simulation, and using a different technique for the pressure inverse step. Besides this, regression of isotherm curve and concentration curve for the transfer function can have a significant impact on the prediction accuracies. Obtaining more data points for regressing the concentration curve can increase the accuracy of the model, but the extra effort would be a trade-off. Nevertheless the simulation results showed a close match to the experimental data under different combinations of parameters. After the model is iteratively solved and converged, the concentrations of rich product and lean product can be obtained. III. RESULTS AND DISCUSSION Before applying the new model to nitrogen and methane separations, it is essential to test the validity of the transfer function model. In order to do so we selected a series of DR-PSA experiments done by McIntyre et al. for separating ethane and nitrogen in 2002 and 2010 for comparison. The main reason for selecting them is that these experiments covered a wide range of different key parameters, such as pressure ratio (defined as the ratio between adsorption pressure and desorption pressure: PA/PD), feed composition (yF), stripping reflux ratio (RS), enriching reflux ratio (RE) and feed step time (tf). We have picked 2 scenarios from the 2002 experiments and 2 scenarios from the 2010 experiments, and their key parameters are listed in Table 1 [8, 9]. Table 1. Key parameters of selected McIntyre et al. experiment scenarios [8, 9]. Case Scenario QF (sccm) PA (bar) PD (bar) yF RS RE tf (s) 1 2002, scenario 2 580.0 2.10 0.27 0.80% 0.82 85 95 2 2002, scenario 3 544.0 2.10 0.27 0.78% 0.56 123 95 3 2010, scenario 1 583.6 1.84 0.26 1.35% 0.38 72 45 Some of the distillation-like characteristics of DR-PSA units can be observed through the experiment result and model predictions with respect to different parameter values. For example, RS was reduced and RE was increased dramatically in case 2 compared to case 1, with tf kept constant. yE increased from 41.0% to 63.9%, suggested by the experiment result and from 38.1% to 59.3%, both showing that decreasing RS and increasing RE will result in increased heavy gas composition rich product while sacrificing the purity in the lean product, which agrees with similar results from distillation columns. Another example was the increased RS and RE in case 4 compared with case 3. RS was increased from 0.38 to 1.57, while RE was increased from 72 to 149. This resulted in a decrease of yL from 2900ppm to 2000ppm and an increase in yE from 61.6% to 70.7%, both indicating purer lean and rich products. As RS and RE can be translated into power input in actual operations, this proved that the purity of products can be elevated by increasing the total power input of DR-PSA units. 4 2010, scenario 6 583.9 1.84 0.26 1.36% 1.57 149 45 where RS and RE are defined as: RS=QLR/QLP (1) RE=QHR/QHP (2) All these scenarios had a feed to low-pressure column configuration, and the column dimensions were given as 0.87m high and 0.0121m radius. Axial feed position was set to be at the middle of the column. MeadWestvaco BAX-1500 activated carbon was used as the adsorbent in all the scenarios, and its adsorption isotherm data were obtained from McIntyre’s paper [8]. The isotherm curves of ethane and nitrogen follow a typical Langmuir isotherm, and they were fitted to Since this is on-going work, we only performed a preliminary run of methane/nitrogen separation after the model was validated using experimental 4 data from the literature. The preliminary run was conducted for the separation of 3% methane and 97% nitrogen. The adsorbent used was Norit RB3 activated carbon. RE and RS were both set to 10, and a total throughput was set at 0.85MMscfd. The column is 20 metres high and 3 metres in diameter which is at industrial scale. A pressure ratio of 3 and 5, feed temperature at 240K and 302K and feed to high/low pressure column configuration were simulated. The detailed results are illustrated in Figure 5. IV. CONCLUSIONS AND FUTURE WORK In our research, a new modelling methodology for DR-PSA units has been proposed. The model combines transfer functions of stripping and enriching PSA units and is iteratively solved according to the material balance of the DR-PSA unit. The validity of the model was verified by comparing the simulation results with the experimental results in the literature in a variety of scenarios which covered a wide range of parameter values. The comparison confirmed that the model is capable of predicting the product concentrations of DR-PSA units as they closely match the experimental results in all scenarios. A preliminary simulation run was done for separating methane and nitrogen in industrial scale. The results were promising as DR-PSA units showed high separation efficiencies in the run. Future work will focus on linking the equilibrium theory model with the transfer function model, as equilibrium theory model is able to optimize the operating parameters and the transfer function model is able to predict the product compositions. Optimization of the transfer function model in methane/nitrogen separations will be also carried out to obtain purer products and lower power consumption. The transfer function model also has the potential to be used in other gas separations, such as CH4/CO2 separations and N2/O2 separations. Figure 5. Results for the comparison of changing pressure ratio, temperature and configuration in terms of yE, yL and RecHP. The parameter combinations are: adsorption pressure (3bar and 5bar), feed temperature (302K and 240K), and DRPSA configuration (feed to high pressure column configuration as HP, feed to low pressure column as LP). It can be observed from Figure 5 that feed to low pressure column configuration can obtain a better result in yL and RecHP, with a minor sacrifice in yE. Either high adsorption pressure (pressure ratio) or low temperature contributed to yL and RecHP, with the lowest yL and highest RecHP obtained in 3bar 240K LP configuration, being 880ppm and 98.7% respectively. We performed simulation runs for adsorption pressure at 5bar and temperature 240K as they seemed to contribute to lowering yL individually, but resulted in an increase in yL compared to the 5bar 302K and 3bar 240K scenario, indicating the large nonlinearity between adsorption pressure and temperature. Due to the low selectivity of the adsorbent and the absence of optimization of the model, the above results still cannot achieve the separation objective. We believe optimization needs to be carried out in search of the best combinations of pressure ratio and temperature, axial feed position, reflux ratios and feed step time. Considering that this is only a preliminary run with no optimization, the result is encouraging and again proved that DR-PSA has the potential for efficient separation, in terms of the low yL and high RecHP. 5 Output Input Table 2. Simulation results and comparison with experimental data QF (sccm) QLP (sccm) QHP (sccm) OT (sccm)# yF yL (ppm) yE RecLP RecHP 2002 Scenario 2 Experiment Model Deviation* 580 555 -4.31% 548 544 -0.73% 11.8 11.0 -6.78% 1010 1010 0.00% 0.80% 0.80% 0.00% <1 467 0.05% 41.0% 38.1% -2.90% 99.9% 98.2% -1.70% 100.0% 94.3% -5.70% 2002 Scenario 3 Experiment Model Deviation* 544 543 -0.18% 538 536 -0.37% 6.8 6.8 0.00% 843 843 0.00% 0.78% 0.78% 0.00% 7 380 0.04% 63.9% 59.3% -4.60% 99.9% 99.5% -0.40% 99.9% 95.2% -4.70% 2010 Scenario 1 Experiment Model Deviation* 583.6 583.3 -0.05% 573.0 573.1 0.02% 10.6 10.3 -3.10% 733.0 722.7 -1.41% 1.35% 1.35% 0.00% 2900 2000 -0.09% 62.6% 63.9% 1.27% 99.2% 99.4% 0.14% 80.8% 83.2% 2.42% 2010 Scenario 6 Experiment Model Deviation* 583.9 583.9 0.00% 574.0 574.0 0.00% 9.9 9.9 0.00% 1483.9 1483.9 0.00% 1.36% 1.36% 0.00% 1900 2520 0.06% 70.7% 65.6% -5.10% 99.5% 99.4% -0.10% 86.9 81.8% -5.10% * The percentage deviations of QF, QLP, QHP and QT are calculated as ((Model-Experiment)/Experiment*100%). For the concentrations and recoveries, the percentage deviations are directly calculated by (Model-Experiment), as they are already in percentage. # QT was defined as the maximum flow rate in the DR-PSA internal material loop. The maximum flow rate value appears in the local feed stream to the first stripping/enriching PSA column downstream of the actual feed, i.e. stream 1 in Figure 2. 6 NOMENCLATURE 3. QF = volumetric feed rate, cm3(STP)min-1 QHR = volumetric flow rate of rich product, cm3(STP)min-1 4. QHP = volumetric flow rate of enriching reflux stream, cm3(STP)min-1 QLR = volumetric flow rate of lean product, cm3(STP)min-1 QLP = volumetric flow rate of stripping reflux, cm3(STP)min-1 5. QT = maximum volumetric flow rate in a DR-PSA unit, cm3(STP)min-1 6. PA = adsorption pressure, bar PB = desorption pressure, bar RS = stripping reflux ratio RE = enriching reflux ratio RecHP = recovery of heavy gas 7. RecLP = recovery of light gas tf = adsorption/desorption step time, s yE = molar fraction of heavy gas in rich product yF = molar fraction of heavy gas in feed 8. yL = molar fraction of heavy gas in lean product REFERENCE 1. 2. 9. BP. Statistical Review of World Energy. 2012 [cited 2013 June]; Available from: http://www.bp.com/assets/bp_inter net/globalbp/globalbp_uk_english/r eports_and_publications/statistical_ energy_review_2011/STAGING/lo cal_assets/pdf/statistical_review_of _world_energy_full_report_2012.p df. BP. Energy Outlook 2010-2030. 2012 [cited 2013 June]; Available from: http://www.bp.com/liveassets/bp_i nternet/globalbp/STAGING/global 10. 11. 7 _assets/downloads/O/2012_2030_e nergy_outlook_booklet.pdf. Rufford, T.E., G. Watson, and E.F. May, Unpublished Work, 2012, The University of Western Australia. Chevron. Gorgon - Our business. 2013 [cited 2013 July]; Available from: http://www.chevronaustralia.com/o urbusinesses/gorgon.aspx. Arthur, K. and P.R. William, Fundamental of Natural Gas Processing. 2006: Taylor and Francis, LLC. Diagne, D., M. Goto, and T. Hirose, New PSA Process with Intermediate Feed Inlet Position Operated with Dual Refluxes: Application to Carbon Dioxide Removal and Enrichment. Chem Eng Japan, 1994. 27(1): p. 85-89. Diagne, D., M. Goto, and T. Hirose, Parametric Studies on CO2 Separation and Recovery by a Dual Reflux PSA Process Consisting of Both Rectifying and Stripping Section. Ind. Eng. Chem. Res., 1995. 34: p. 3093-3089. McIntyre, J.A., C.E. Holland, and J.A. Ritter, High Enrichment and Recovery of Dilute Hydrocarbons by Dual-Reflux Pressure-Swing Adsorption. Ind. Eng. Chem. Res., 2002. 41: p. 3499-3504. McIntyre, J.A., A.D. Ebner, and J.A. Ritter, Experimental Study of a Dual Reflux Enriching Pressure Swing Adsorption Process for Concentrating Dilute Feed Streams. Ind. Eng. Chem. Res., 2010. 49: p. 1848-1858. Ebner, A.D. and J.A. Ritter, Equilibrium Theory Analysis of Dual Reflux PSA for Separation of a Binary Mixture. AIChE Journal, 2004. 50(10): p. 2418-2429. Kearns, D.T. and P.A. Webley, Modelling and evaluation of dualreflux pressure swing adsorption 12. cycles: Part I. Mathematical models. Chemical Engineering Science, 2006. 61: p. 7223-7233. Kearns, D.T. and P.A. Webley, Modelling and evaluation of dual reflux pressure swing adsorption cycles: Part II. Productivity and energy consumption. Chemical Engineering Science, 2006. 61: p. 7234-7239. 8