A Genetic Animal Model Of Bipolar Disorder
advertisement

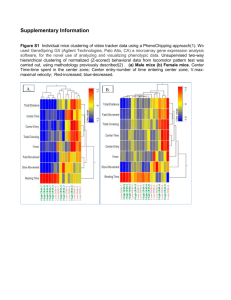
Convergent Functional Genomics Studies In Mice Lacking The Circadian Clock Gene DBP: A Genetic Animal Model Of Bipolar Disorder Y. Balaraman1, 2,3*, H. Le-Niculescu1, 2,3*, C. Ogden5*, J. Tan1, 3*, S. Patel1,3, , M. E. Rich5, J.B. Lohr5, I. Nicastro5, M. Paulus5, H.J. Edenberg4, J. I. Nurnberger3, U. Schibler6, M. Geyer 5, S. V. Faraone7, M. T. Tsuang5 , R. Kuczenski 5 and A. B. Niculescu1, 2, 3 ¶ 1 Laboratory of Neurophenomics, 2INBRAIN, 3Institute of Psychiatric Research, Indiana University School of Medicine, 791 Union Drive, Indianapolis, IN 46202; 4Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, 635 Barnhill Dr. Indianapolis, IN 46202; 5Department of Psychiatry, UC San Diego, 9500 Gilman Drive, La Jolla, CA 92093; 6Department of Molecular Biology, University of Geneva; 7 Department of Psychiatry, SUNY Upstate Medical University,750 East Adams St., Syracuse, NY 13210. * authors contributed equally to this work ¶ Corresponding author, E-mail: anicules@iupui.edu Running title: A Genetic Animal Model Of Bipolar Disorder Keywords: clock gene; mouse; knockout; methamphetamine; body weight; stress; bipolar disorder; microarrays; brain; genetics Abstract: We had previously identified the clock gene D-box Binding Protein (DBP), a transcription factor, as a potential candidate gene for bipolar and related disorders, using a Convergent Functional Genomics approach (Niculescu et al. 2000) that crossmatched gene expression data from a methamphetamine treated rodent model with human genetic linkage data. Here we report that mice with a homozygous deletion of DBP (DBP KO) (Lopez-Molina and others 1997) have a baseline lower locomotor activity, blunted responses to acute methamphetamine administration, and gain less weight over time compared to wild-type controls, analogous to DSM criteria of depression in humans. In response to a chronic stress paradigm, DBP KO mice exhibit a diametral switch in these phenotypes. Taken together with previous data showing that DBP KO mice have abnormal circadian and homeostatic aspects of sleep regulation (Franken and others 2000), our work suggests they reproduce phenotypic aspects of bipolar disorder. Microarray studies of brains of both non-stressed and stressed mice reveal a pattern of gene expression changes that may explain the observed phenotypes, and implicate DBP as a potential master switch gene in bipolar disorder pathophysiology. Convergent Functional Genomics (CFG) analysis of the gene expression changes reproduced top previous findings by us (Ogden and others 2004) and others in the field using different approaches, as well as identified novel candidate genes for bipolar disorder. We propose that mice lacking DBP may be a useful genetic animal model of bipolar and related disorders.








![The Effect of Dibenzo[a,l]pyrene on the Thymus of Fetal Mice Vivian LaRonge](http://s2.studylib.net/store/data/015050646_1-3fc452edb9b3725306be7cd2cf015d28-300x300.png)