EXP. 4 - UniMAP Portal

EXPERIMENT 4
EFFECT OF CELL IMMOBILIZATION ON DYE REMOVAL BY
BIOABSORBENT
1.0 OBJECTIVES
1.1 To immobilize activated carbon with fungal biomass for bioabsorbent.
1.2 To analyze the effect of cell immobilization on methylene blue dye removal by bioabsorbent.
2.0 COURSE OUTCOMES
CO2-Ability to evaluate microbial system based on its metabolic pathways and kineticsstudy in batch and continuous cultures.
3.0 INTRODUCTION
Dyes are widely use in textile, paper, plastic, food and cosmetic industries. The wastes coming from these industries can effect on our atmosphere causing pollution. The level of pollutants even in very low concentration is highly visible and will affect aquatic life as well as food web. Many dyes are difficult to degrade. They are generally stable to light, oxidizing agents and are resistant to aerobic digestion. Hence, contaminations due to dyes pose not only a severe public health concern, but also many serious environmental problems because of their persistence in nature and non-biodegradable characteristics. The numbers of conventional methods are available for color removals from industrial effluents including ion exchange, adsorption, membrane technology and coagulation. Among all the sorption process the activated carbon has been shown to be one of the most efficient adsorbent for the removal of dyes from effluents.
4.0 MATERIALS AND EQUIPMENTS
4.1
4.2
4.3
4.4
4.5
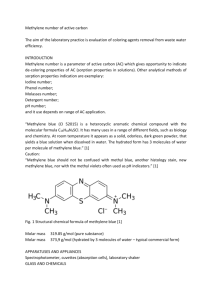
Methylene blue
Activated carbon
Sodium hydroxide (NaOH)
Hydrochloric acid (HCl)
Potato dextrose agar
4.6
4.7
4.8
4.9
4.10
4.11
4.12
Distilled/sterilized water
Test tubes and flasks
Water bath
Pipettesand beakers
Hot plate and magnetic stirrer
Spectrophotometer
Incubator shaker
5.0 PROCEDURES
5.1 Preparation of fungal biomass
5.1.1 Culture the Phanerochaete chrysosporium at 32 o C for 7 days on potato dextrose agar plates.
5.1.2 Scrape the spores on agar plates into sterile water to form a spore suspension.
5.1.3 Add 1 ml of the spore suspension per 100 ml of culture medium containing malt extract and activated carbon (at initial pH of 5) which is previously autoclaved at 121 o C for 15 minutes. The culture medium without activated carbon is set as a control. Different concentrations of activated carbon are used for preparation of fungal biomass.
5.1.4 Incubate the culture medium at 32 o C and 150 rpm for 2 days.
5.1.5 After 2 days, collect the mycelia balls and keep in an oven at 80 o C for
6 hours to prepare the fungal biomass.
5.1.6 Grind the fungal biomass using a mortar and a pestle into powder.
5.2
5.3
Preparation of dye solution
5.2.1 Prepare the stock solutions of methylene blue at different concentrations with distilled water.
5.2.2 Adjust the desired pH values of dye solutions with 0.1 NaOH or HCl solution using pH meter.
Batch biosorption studies
5.3.1 For the samples, use 0.1 g of fungal biomass powderwith 50 ml of methylene blue solutions at concentration of 20 mg/l and pH 10.
5.3.2 Shake samples at 150 rpm in incubator shakerat 30 o C for 120 minutes.
5.3.3 Take out the samples from the incubator shaker and filter the samples to remove the fungal biomass.
5.3.4 Analyze the supernatant to determine the concentrations of remaining
5.3.5 Determine the concentrations of methylene blue in the supernatant using a standard curve. methylene blue in the solution using spectrophotometer set at a wavelength of 664 nm.
6.0 RESULT
6.1 Construct a standard curve for the methylene blue dye from the stock solutions.
Dye concentration
(mg/l)
Absorbance
(Abs at 664 nm)
0
10
20
30
6.2
40
50
Record your result into a table below and then determine the dye concentrations (C e
)based on your methylene blue standard curve.
Contact time
(min)
0
Absorbance
(Abs at 664 nm)
C e
(mg/l)
30
60
90
120
6.3 Calculatethe removal efficiency (%) of methylene blue dye at different concentrations of activated carbon immobilized with fungal biomass over time.
6.4 Calculate the amount of adsorption at equilibrium, q e
(in mg/g), where q e
= (C o
-C e
)V/W, where C o is initial concentration (in mg/l), C e
is concentration of dye in solution at any time (in mg/l), W is the weight of biomass (g) and V is the volume of dye solution (l)
6.5 Plot the equilibrium data using Freundlich (ln q e
vs ln C e
) and
Langmuir (C e
/q e
vs C e
) isotherms, respectively.
7.0 DISCUSSION
Discuss in detail the obtained results by relating them with theory which you have learned in class.
8.0 CONCLUSION
Draw some conclusions based on the experimental procedures done and the results obtained in this experiment
9.0 QUESTIONS
10.0 REFERENCE
African Journal of Biotechnology Vol. 9(6), pp. 874-881, 8 February, 2010