Experiment No 2
advertisement

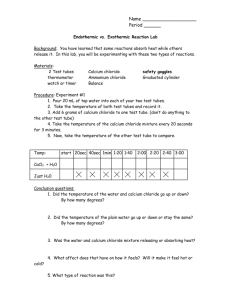
PRACTICAL EXPERIMENTS EXPERIMENT NO.1 Aim: To determine the melting point of ice and the boiling point of water. (a) To determine the melting point of ice. MATERIALS REQUIRED: Beaker, wire gauze, tripod stand, burner, thermometer, stirrer,ice cubes,thermometer,clamp stand etc.. THEORY: The constant temperature at which a solid changes into a liquid is called melting point of the solid. When a solid is heated, its molecules absorb heat energy and their kinetic energy increases. This results in rise of temperature of the solid. During melting, the temperature remains the same till the entire solid melts even though we continue to supply the heat. The quantity of heat required to completely change 1 kg of ice into water without any change in temperature is known as latent heat of fusion of ice. PROCEDURE: Take some ice cubes in a beaker and place it over a wire gauze kept over a tripod stand. Suspend a thermometer with the help of a clamp stand so that its bulb is in contact with the ice. Start heating the beaker on a low flame and note the temperature when the ice starts melting. Finally note the temperature when all the ice has been converted into water. OBSERVATION: The temperature remains constant till the ice has completely melted i.e 0o Celsius. RESULT: The melting point of ice is 00 C . PRECAUTIONS: 1. The bulb of the thermometer should be completely surrounded by ice. 2. Ice should be stirred regularly to keep a uniform temperature throughout. 3. Note temperature by keeping your eyes in line with the level of mercury. (b) To determine the boiling point of water. MATERIALS REQUIRED: Distilled water, boiling tube, rubber cork with two holes, delivery tube, clamp stand, thermometer, pumice stones etc. THEORY: When heat energy is supplied to water, the particles start moving faster. At a certain temperature, a point is reached when the particles have enough energy to break free from the forces of attraction of each other. At this temperature the liquid starts changing into gas. The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point. During the change of state from liquid to gas, the temperature of the system remains the same till the entire liquid is converted into vapours even though heat is continuously being supplied. The amount of heat energy required to change 1 kg of liquid into vapours at atmospheric pressure at its boiling point is called latent heat of vapourisation. PROCEDURE: Take some distilled water in a boiling tube and add few pieces of pumice stones to it. Fix a cork with two holes(one for thermometer and other for delivery tube) in the mouth of the boiling tube and clamp it with the stand. The thermometer should be introduced in such a way that the bulb of the thermometer is 3-4 cm above the surface of water. Heat the boiling tube and note the temperature when the boiling starts. Continue to heat till the temperature becomes constant and the water remains boiling. Note the constant temperature. OBSERVATION: The temperature remains constant till all the water has been converted into vapour (i.e at 100oC.) RESULT: The boiling point of water is 100oC Plot a graph of Temperature vs Time (in minutes), . PRECAUTIONS: 1. The bulb of the thermometer should be kept 3-4 cm above the surface of water 2.|Pieces of pumice stone should be added to water before heating to avoid bumping. 3. Heating of water should be done by rotating the flame. 4. Note temperature by keeping your eyes in line with the level of mercury. . EXPERIMENT NO.2 Aim: To separate the components of a mixture of sand, common salt and ammonium chloride (or camphor) by sublimation. Requirement: Common salt, sand and ammonium chloride, distilled water, china dish, funnel and tripod stand. Principle: Constituents of a mixture can be separated by simple physical methods. Sublimation: It is the conversion of a solid directly into the vapour phase without changing to a liquid. Sublimation can be used to separate a volatile solid and a non-volatile solid in a mixture. The water-soluble solid/substance can be separated from non- water-soluble substance by filtration. Experimental steps: Mix the salt, sand and ammonium chloride thoroughly to get a mixture. Take some amount of the mixture in a china dish; place it on a tripod stand for heating. Cover the china dish with an inverted funnel with some cotton plugged in its stem to prevent vapours escaping out. Continue heating for sometime until no more vapours of ammonium chloride are seen to be rising up. Remove the funnel carefully so that the ammonium chloride deposited does not fall. The mixture of sand and common salt remains in the china dish. Cool the contents of the china dish and dissolve in a minimum amount of water and filter. Sand remains as residue; the filtrate is concentrated by evaporation and left undisturbed for salt to crystallize out. Conclusions: Ammonium chloride is a sublimable substance, which is first separated by subliming the mixture. Common salt readily dissolves in water and is separated from sand by dissolving in water and filtering the mixture. Residue obtained is only sand. Constituents of the given mixture could be separated by simple physical methods. Precautions: While heating the mixture the china dish should be very stable on the wire gauze over the tripod stand. The funnel should not be very big otherwise vapours will escape out. The stem of the funnel should be plugged with cotton to prevent loss of ammonium chloride vapours. Water should not be added to the contents of the china dish unless it has cooled down. Ammonium chloride should be gently scraped off from the funnel once it has cooled to room temperature. Experiment No. 3 To prepare a) a true solution of common salt, sugar and alum b) a suspension of soil, chalk powder and fine sand in water c) a colloidal of starch in water and egg albumin in water and distinguish between these on the basis of i) Transparency ii) Filtration criterion iii) Stability Requirements: common salt, sugar, alum, soil, chalk powder, fine sand, starch, distilled water egg albumin, test tubes, funnel, filter paper, test tube stand, glass rod, beakers. Theory: Solution is a homogeneous material formed by mixing of two substances, one in large amount (solvent) and the other in small amount (solute) . The particle size is smaller than 10-7 cm. The solution in which no more solute is soluble in the solvent at a given temperature and pressure conditions is said to be a saturated solution. A suspension is a heterogeneous material in which the solid particles can be seen by naked eye and magnifying glass. The suspended particles can be separated by filtration or by sedimentation as the particles settle down on standing. The particle size is of the order of 10-5 cm or larger. A colloid is a heterogeneous material. The particles of a colloidal system can be seen by a powerful microscope. A colloidal system is stable; its components do not settle down under gravity and components cannot be separated by filtration. The particle size is generally in between 10-7 to 10-5 cm. Procedure: a) Preparation of a true solution of common salt, sugar and alum: Take three beakers marked A, B and C containing 90ml of water in each. Add 10g of fine powder of each common salt, sugar and alum in beakers A, B and C respectively. Stir the solution of each beaker thoroughly with the help of a glass rod. b) Preparation of a suspension of soil, chalk powder and fine sand in water : Take three beakers marked D, E and F each containing 90ml of water. Add 10g of fine soil, fine chalk powder and fine sand in each of the beakers respectively. Stir the contents of each beaker well with a glass rod. c) Preparation of a colloidal solution: i) a colloidal of starch in water: Mix 1% dry cornstarch with 3ml of distilled water. To this add 97ml of boiling distilled water and stir it well. Cook it for two minutes stirring the solution continuously. Cool it and store in a test tube marked G. ii) a colloidal of egg albumin in water : Take 1g of egg albumin and 5ml of distilled water in a beaker and mix it well. Slowly add 95ml of distilled water while stirring constantly. After mixing add a few drops of dil.HCl or dil.H2SO4 and stir well. The clear solution of albumin and water will become turbid. Store this in a test-tube marked H. Property to be tested 1.Transparency 2. Filtration 3. Stability Experiment Observation Inference Paste small strips of same coloured cellophane paper on one side of each test-tube (A, B, C, D, E, F, G and H). Arrange test-tubes in groups according to solution, suspension and colloid. Now observe the coloured paper of each test-tube from the other side of the test-tube through the liquid one by one. Filter the contents of testtubes A, B and C separately. Colour spot is clearly sen from the other side of the test-tubes A, B and C A true solution is transparent while colloids and suspensions are not. No residue is left on the filter paper. A clear filtrate is obtained. Solid particles cannot be separated from true solution by filtration. Filter the contents of testtube D, E and F separately. Particulate suspension is seen on the filter paper in each case but filtrate is a clear liquid. Suspended components of a suspension can be separated by filtration. Filter the contents of testtubes G and H separately. No residue left on the filter paper in both the cases. But the filtrate obtained is translucent. Components of a colloid cannot be separated by filtration. Leave the test-tubes A, B, C, D, E, F and G for sometime. No change in test tubes A, B and C. the solution remains as it is without any settlement. In test tubes D, E, F there is a gradual settlement of solid particles at the bottom. The true solutions are stable and do not show deposition of components. Suspensions are unstable and show settlement of heavier particles. No change is observed in test-tubes G and H Colloids are stable. Their solute particles do not settle down after sometime. Result: (a) Colloidal solutions are somewhat translucent and their particles can pass through filter paper to give translucent filtrate. No particles are left as residue on the filter paper. (b) True solutions are transparent; they pass through filter paper leaving no residue on the filter paper. The filtrate is also transparent. (b) Suspension is opaque or dull. They leave residue particles over filter paper on filtration. The filtrate is more or less clear and transparent. EXPERIMENT NO.4 Aim To prepare a) a mixture b) a compound using iron filings and sulphur powder and distinguish between these on the basis of : i) Appearance i.e., homogeneity and heterogeneity ii) Behavior towards a magnet iii) Behavior towards carbon disulphide a solvent. iv) Effect of heat. Apparatus required: A hard glass tube; test tube holder; pestle and mortar, two watch glasses, a hand lens, a magnet, a rack full of clean test tubes, burner. Chemicals required: Iron filings (10g), sulphur powder (5g), carbon disulphide Theory: Physical Change-Changes in which original components do not change their properties and no new substances are formed. Chemical Change- Changes in which original components undergo change to form new substances with entirely different properties. Procedure: a) Preparation of a mixture of iron and sulphur Take the entire amount of iron filings and sulphur powder and put them in pestle and mortar. Grind the constituents thoroughly. The product so formed is a mixture of iron and sulphur. Divide the mixture into two halves and place them on two watch glasses. b) Preparation of the compound of iron and sulphur (iron sulphide) Transfer half of the mixture from one of the watch glasses to a hard glass test tube. Hold the test tube with the test tube holder and heat the mixture strongly on a Bunsen burner till its contents start glowing with a reddish glow. Stop heating, the test tube will continue glowing for sometime because iron reacts with sulphur to form its compound iron sulphide with the release of heat energy. When the contents of the test tube cool, break the tube and gently remove the pieces of broken glass. Transfer the compound formed in the pestle and mortar and grind well. Now transfer the powdered compound onto the watch glass. Fe + S FeS Procedure: Experiment 1.Action with bar magnet: Roll the bar magnet in the mixture as well as its compound. 2. Appearance: Observe the mixture as well Observations a) In case of mixture, iron particles cling to the magnet. b) In case of its compound The black particles do not cling to the magnet. a) In case of mixture the grey particles of iron can be seen Inference a) Constituents of a mixture retain their properties and can be separated by physical means. b) But constituents of a compound can not be separated by physical means a) Mixtures are heterogeneous in nature as its compound under magnifying glass by spreading them thinly on a paper. 3. Action with carbon disulphide: Place a small amount of the mixture and its compound in separate test tubes and add 5 ml of carbon disulphide and shake them well. 4. Action of heat: Take small amount of mixture and compound in different test tubes respectively and heat them on the Bunsen flame. clearly in yellow particles of sulphur and they are not uniform throughout. b) In case of the compound, uniform black particles are seen. a) In the case of mixture the yellow particles of sulphur dissolve and black particles of iron settle down. b) In the case of compound no change occurs. a) In case of mixture the test tube starts glowing with a reddish glow. When the heating is stopped the reddish glow will continue for sometime. A grey solid mass is formed. b) In case of compound no visible change occurs b) Compounds are homogeneous in nature. a) Sulphur retains its properties in the mixture. b) Sulphur does not retain its properties in the compound. a) On heating, mixture of iron and sulphur reacts and forms iron suphide. b) No reaction takes place on heating iron sulphide Result: i) When iron filings and sulphur powder are mixed, both retain their properties. It means they have not undergone any chemical reaction. Thus it is a physical change. ii) When iron filings and sulphur powder are mixed and heated, they undergo a chemical reaction. A new substance iron sulphide is formed which has properties entirely different from iron and sulphur. Thus it is a chemical change. PRECAUTIONS: 1. Heat the mixture of iron and sulphur in a hard glass tube only. 2. Remove the pieces of broken glass with forceps only. Do not use bare hands as you are likely to injure your fingers. 3. Carbon disulphide should be kept away from the flame (It is volatile and can catch fire). 4. Hydrogen sulphide should not be inhaled. It can cause a headache. Experiment No. 5 Aim: To carry out the following chemical reactions and record observations. Also to identify the type of reaction involved in each case. i) Burning of Magnesium in air. ii) Zinc with dilute sulphuric acid iii) Iron with copper sulphate solution in water. iv) Heating of Lead Nitrate. v) Sodium sulphate with Barium chloride in the form of their solutions in water. EXPERIMENT NO.5A Aim: Burning of Magnesium in air. Requirements: a) A strip of magnesium ribbon, tongs, china dish, burner. Theory: Magnesium is a reactive metal; it combines with oxygen to form an oxide. Combination reaction: It is a reaction in which two elements combine to give a compound e.g. 2 Mg + O2 2 MgO magnesium oxide Magnesium forms a basic oxide as it dissolves in water to form magnesium hydroxide. MgO + H2O Mg(OH)2 Magnesium hydroxide Magnesium hydroxide turns red litmus blue. S.No. Experiment 1 Take a clean strip of It burns with a dazzling flame magnesium ribbon and hold forming a white powder. it with the tongs. Burn it the flame of burner. Collect the white powder Red litmus paper turns blue. in the china dish and dissolve it in distilled water and dip a red litmus paper in it. 2. Observations Inference Magnesium combines with oxygen to form Magnesium oxide. Magnesium oxide is basic in nature. Precautions: The magnesium ribbon should be cleaned before the experiment because it is a reactive metal; it combines with oxygen in air to form an oxide. The dazzling flame should not be seen directly for long time. The strip of magnesium ribbon should be held with a tongs to protect our hands from burning. The white magnesium oxide powder should not be touched by hand. EXPERIMENT NO.5B Aim: Reaction of Zinc with dilute sulphuric acid Requirements: Piece of zinc metal, dilute sulphuric acid, test tubes. Theory: Zinc is very reactive metal and can displace hydrogen from dilute acids. Displacement reaction: When metals like Zn, Mg, Fe, more reactive than hydrogen, react with dilute acid hydrogen gas is liberated e.g. Zn(s) + H2SO4(aq) ZnSO4(aq) + H2(g) This is also an example of a Redox reaction ie reaction in which oxidation and reduction are taking place simultaneously. S.No. 1. 2. 3. Experiment Take a few pieces of zinc in the test tube and add about 20ml of dil. H2SO4 to it. Bring wet red and blue litmus papers near the mouth of the test tube. Close the mouth of the test tube with your hand to prevent the gas from going out and then bring a burning matchstick near the mouth of the test tube. Observations Bubbles are formed immediately showing gas being evolved No colour change is observed on either red or blue litmus papers. The matchstick goes off with a ‘pop’ sound but the gas burns with a blue flame. Inference Hydrogen evolved. gas is Hydrogen gas is neither acidic nor basic. Hydrogen gas does not support combustion but is combustible. Precautions: Dil. Sulphuric acid should be handled with care. Zinc granules should be cleaned before adding them to acid. Test for hydrogen gas with a matchstick should be done carefully. EXPERIMENT NO.5C Aim: Iron with copper sulphate solution in water. Requirements: Iron nail, copper sulphate, distilled water. Theory: Iron is more reactive than copper as it is above copper in the reactivity series. Displacement reaction: It is a reaction in which a more reactive metal displaces a less reactive metal from its salt solution. Reaction: Fe + CuSO4 FeSO4 + Cu (blue) (green) S.No. Experiment 1 Clean an iron nail and put it a test tube.Add about 10 ml of copper sulphate solution to it and leave it for some time. Observation After 15 minutes the colour of the solution changes from blue to light green and a brown coating is observed on the surface of the nail. Inference The brown coating on the nail shows that copper from the copper sulphate solution has been displaced by iron and green coloured ferrous sulphate has been formed. Iron is more reactive than copper Precautions: Iron nail should be thoroughly cleaned. A clean nail and some solution of copper sulphate should be kept aside for comparison. During the experiment the test tube should not be disturbed. EXPERIMENT NO.5(D) Aim: Sodium sulphate with Barium chloride in the form of their solutions in water. Requirements: Sodium sulphate solution, barium chloride solution, conical flask and glass rod. Theory: Double displacement reaction: A reaction in which exchange of two metal atoms takes place simultaneously, e.g. BaCl2 (aq) + Na2SO4 (aq) S.No. Experiment 1 Take 10 ml each of sodium sulphate and barium chloride solutions in different test tubes. Mix the two solutions. BaSO4(s) (White ppt) Observation A white precipitate is formed + 2NaCl (aq) Inference A double displacement reaction takes place. A white precipitate of barium sulphate is formed and sodium chloride remains in solution Precautions: All glass apparatus has to be handled with care. EXPERIMENT NO.5 (F) Aim: Heating of Copper sulphate crystals. Requirements: Copper sulphate crystals, hard glass test tube, burner, test-tube holder. Theory: Copper sulphate decomposes and forms water vapour, leaving white anhydrous copper sulphate. The compound will become white in colour as the water evaporates. This compound is still copper II sulphate but in dehydrated form.This reaction is reversible. If water is added to anhydrous copper sulphate then the solution turns blue again accompanied by a rise in temperature. heat CuSO4.5H2O ----------- CuSO4 + 5H2O On very strong heating (almost above 250ºC), the anhydrous the copper sulfate decomposes to form copper oxide. CuSO4 (aq) ==> SO2(g) + CuO(s) Decomposition reaction: A compound breaks down to form two or more compounds. Experiment Take a small amount of copper sulphate crystals in a dry testtube. Hold the test-tube with a test-tube holder. Heat the testtube over the flame of a burner first gently and then strongly. Observation Water droplets are collected at the cooler sides of the test tube. The blue colour disappears and the crystals become a white powder. On very strong heating, a black solid remains in the test tube. Inference The crystals lose the water of crystallization on heating. The crystalline shape and blue colour is lost due to loss of water of crystallization. CuSO4 decomposes to form copper oxide. Precautions: i) Keep the mouth of the test-tube away from your face and also from other classmates. ii) Always use test-tube holder while heating the test-tube. iii) Use pyrex hard glass test tubes as the normal ones may crack with the heat supplied. TESTING OF PRACTICAL SKILLS IN SCIENCE & TECHNOLOGY Experiment No 1 Aim: To determine the melting point of ice and the boiling point of water. 1. The temperature at which solid starts melting is called: a) Freezing point b) Melting point c) Boiling point d) Critical point 2. Unit of temperature is : a) Celsius b) Kelvin c) Fahrenheit d) All of the above 3. Boiling is a : a) Slow process b) Noisy process c) Rapid process d) Independent process 4. Heat is liberated when : a) Water boils b) Ice melts c) Temperature of water is increased d) Vapours condense 5. At the stage when water starts converting itself into steam, the temperature : a) Remains constant b) Continuously increases c) Decreases d) Can’t be observed 6. The boiling point of a liquid depends on the : a) Volume of liquid b) Pressure exerted on its surface c) Climate d) Both (a) and (b) 7. The value of the melting point of a substance and freezing point have : a) Different values b) Same values c) Both (a) and (b) d) None of the above 8. When all ice floating in water melts, the level of water in the container : a) Falls b) Rises c) Remains the same d) None of the above 9. the name of A, B, C and D in the following diagram are : A ------------ ----------D Solid a) b) c) d) Liquid B ---------- Gases -------C A – Fusion, B – Vaporization, C – Condensation, D – Solidification A – Vaporization, B – Fusion, C – Condensation, D – Solidification A – Condensation , B – Vaporization, C – Solidification, D – Fusion A – Solidification, B – Vaporization, C – Fusion, D – Condensation 10. Water evaporates faster : a) In still air b) In humid air c) In dry air d) In windy and dry air Scoring Key Experiment No. 5 Q.No. 1 Correct choice b 2 3 4 5 6 7 8 9 10 d c d a b b c a d Explanation The process of conversion of a solid to liquid is called melting & the temperature as melting point All are units of temperature on different scales Boiling is a fast visible process When vapours condense into a liquid heat is released During change of state the temperature remains constant An increase in pressure on the surface of water raises its boiling point Melting point of ice is also called freezing point Water is displaced just when ice is dropped Both these increase the rate of evaporation Experiment No 2 Aim: To Separate a mixture of common salt, ammonium chloride and sand. 1. A student heated a mixture of sand and 2 chemical substances which do not react chemically. In a few minutes the mixture started giving white fumes which condensed on a cool glass plate to form white powdery mass. This phenomenon is due to (a) decantation (b) evaporation (c) Sublimation (d) distillation 2. In the above mixture in Q.1, the white powdery substance is commonly called (a) sublimate (b) filtrate (c) Distillate (d) residue 3. A mixture consists of powdered chalk, common salt, sand and camphor. The component which can be separated by just heating is (a) chalk (b) common salt (c) sand (d) camphor 4. A mixture containing ammonium chloride and common salt is heated in a china dish so as to recover ammonium chloride from it. An inverted funnel is placed over the china dish (a) Before heating it (b) after heating it (c) When fumes of ammonium chloride start coming from the mixture (d) When fumes of ammonium chloride stop coming 5. A mixture sand, ammonium chloride and sodium chloride is dissolved in water and then filtered. The filtrate consists of (a) ammonium chloride solution (b) sodium chloride solution (c) sodium chloride and ammonium chloride solution (d) sand and ammonium chloride solution 6. Observe the experimental setup shown below. The components of which mixture can be separated by it? (a) sand and common salt (b)sand and camphor (c) chalk powder and common salt (d) sulphur and sand 7. The sublimate substance used to protect warm clothes (a) benzoic acid (b) iodine (c) naphthalene (d) camphor 8. When a mixture of marble and 2 chemical substances which do not chemically react is heated, the mixture starts giving violet coloured vapours. These vapours condense on a cold glass plate to form black deposits of (a) sulphur (b) sodium chloride (c) iron (d) iodine 9. The following experimental setup was used to demonstrate sublimation. Q. No. Key Explanation The error in the setup is (a) funnel is inverted (b) china dish is used (c) stem of funnel is unplugged (c) the burner is lit 10. Four students were given the following mixtures. Student A:sand and common salt Student B: ammonium chloride and sand Student C: common salt and potassium chloride Student D: marble powder and sand In which of the above cases dense white fumes are formed (a) A (b) B (c) C (d) D 1 (c) Substances can only change from solid to gaseous state during sublimation. 2 (a) 3 (d) The solid state which is directly formed from the gaseous state on cooling is called sublimate. Camphor can easily sublime. 4 (c) More crystals will condense on the cool surface of the funnel. 5 (c) Sodium chloride and ammonium chloride are soluble in water. 6 (b) Camphor can easily sublime. 7 (c) Easily sublimes without leaving any residue. 8 (d) Only iodine sublimates to form black deposits. (c) To prevent the loss of ammonium chloride vapours. (b) Ammonium Chloride is the only substance amongst the mixtures that sublimes. 9 10 Scoring Key Experiment No. 3 Experiment No 3 Aim: To Prepare a) A true solution of common Salt and alum b) A suspension of soil, chalk powder and fine sand in water c) A colloidal of starch in water and egg albumin in water and distinguish between these on the basis of i) Transparency ii) Filtration criterion iii) stability 1. To prepare a colloidal solution of starch, we should: a) Add starch powder to boiling water and cool. b) Add starch powder to cold water and boil. c) Heat starch powder, add it to cold water and boil. d) Add a thin paste of starch to boiling water while stirring. 2. A student was asked to mix the white of an egg with water and stir well. The student observed that: a) A transparent solution is formed b) A translucent mixture is formed c) Egg white settles down at the bottom d) Egg white floats on the surface of water 3. Which of the following is not a colloidal solution? a) Starch in water b) Gum in water c) Soap solution d) Sulphur in carbon-di-sulphide 4. The following substances are added to water in a beaker as shown below. The mixture is stirred well. A true solution is found in the beaker a) A b) B c) C d) D 5. Which one is not a correct statement about a colloidal of starch in water? a) Its particles cannot be seen by naked eyes b) Its components cannot be separated by filtration c) It is unstable d) It does not show Tyndall effect 6. Which of the following is the correct procedure for filtration? a) A c) C b) B d) D 7. The liquid should be poured into the filter cone with the help of: a) Test tube b) Glass rod c) Glass rod pressed against the edge of cone d) The glass rod in the centre of cone 8) Take three test tubes A,B and C containing salt solution, egg albumin in water and suspension of sand in water. Paste small strips of coloured papers on one side of each test tube. Observe the coloured paper from the other side of the test tube through the liquid one by one. Identify the correct observation out of the following: a) Coloured spot is clearly seen in A, appears dim in B and not visible in C. b) Coloured spot is not visible in A, appears dim in B, visible in C c) Coloured spot is visible in A, not visible in B, appears dim in C d) Coloured spot is not visible in A, appears dim in B, not visible in C 9. Take three test tubes A,B and C containing salt solution, egg albumin in water and suspension of sand in water. Filter the contents of A, B and C through filter paper and observe the residue and filtrate. Identify the correct statements: a) A clear filtrate and no residue in A, translucent filtrate and no residue in B,solid particles as residue and clear filtrate in C. b) No residue and clear filtrate in all the test tubes c) Translucent filtrate in all d) Residue left and clear filtrate in all 10. You have prepared four different mixtures in water using charcoal powder,chalk powder, slaked lime and detergent powder. If you filter these mixtures through a filter paper,there will be no residue left after filtration in the case of: a) Charcoal powder b) Chalk powder c) Slaked lime e) Detergent powder SCORING KEY Experiment No.4 Q NO. 1. 2. 3 4. 5. KEY (d) (b) (d) (b) 6. (d) 7. 8. (c) (a) 9. (a) 10. (d) EXPLANATION Thin paste of starch forms colloidal solution. Egg white forms colloids. Sulphur in carbon-di-sulphide forms a true solution. Sugar makes a true solution. The suspension to be filtered should be added into funnel fitted with the help of glass rod and the stem of funnel should touch the sides of the beaker so as to make filtration faster. The suspension to be filtered should be added into funnel fitted with the help of glass rod and the stem of funnel should touch the sides of the beaker so as to make filtration faster. It helps the suspension to reach the cone without spilling. Salt solution is transparent, egg albumin in water is transluscent, suspension of sand in water is opaque. Salt solution passes through filter paper and clear filtrate is observed. Egg albumin in water passes through filter paper but filtrate is translucent whereas in suspension, sand is left as residue and filtrate is clear. Detergents form colloidal solution in water Experiment No. 4 Aim: To prepare a) a mixture b) a compound using iron filings and sulphur powder and distinguish between these on the basis of : i) appearance i.e. homogeneity and heterogeneity ii) behaviour towards a magnet iii) behaviour towards carbon disulphide a solvent. iv) effect of heat. 1. To prepare iron sulphide, by heating a mixture of iron filings and sulphur powder, we should use (a) copper dish (b) watch glass (c) hard test tube (d) Petri dish 2. A student by mistake mixed iron filings and sulphur powder. He wants to separate them from each other. The method you would advise him to use is to dissolve the mixture in (a) boiling water (b) cold water (c) carbon disulphide (d) warm water 3. When we start heating a mixture of iron and sulphur, we observe that (a) sulphur starts melting (b) iron filings start melting (c) mixture becomes red hot (c) mixture evaporates 4. The colors of iron filings and iron sulphide are ((a) blue, black (b) blue, blue (c) black, blue (d) black, black 5. The black compound obtained on heating Fe and S is (a) FeS (b)FeS2 (c) Fe2S2 (d) Fe2S3 6. If a lead acetate paper is brought near H2S it turns black due to (a) formation of a compound (b) formation of a mixture (d) absorption of heat 7. The smell of H2S gas is (a) pleasant (b) fishy 8. Sulphur is soluble in (a) CS2 (b) H2O (c) odourless (c) H2SO4 (d) of rotten eggs (d) both (b) & (c) 9. The reaction between iron & sulphur is accompanied by (a) evolution of light (b) absorption of heat (a) & (c) SCORING KEY Experiment No. 1 (c) no reaction (c) release of heat (d) both Q NO. 1. 2. 3 4. 5. 6. 7. 8. 9. KEY (c) (c) )a( )d( )a( )a( )d( )d( )c( EXPLANATION it is exothermic reaction Sulphur is soluble in CS2 observation both iron fillings and iron sulphide are black Fe + S FeS PbS is formed (black in colour) observation Sulphur dissolves readily in CS2 it is an exothermic reaction EXPERIMENT NO. 5 Aim : To carry out the following chemical reactions and record observations. Also to identify the type of reaction involved in each case – (i) Iron with copper sulphate solution in water (ii) Burning of magnesium in air (iii) Zinc with dilute sulphuric acid (iv) Heating of hydrated copper sulphate (v) Sodium sulphate with barium chloride in the form of their solutions in water 1. The colour of hydrated copper sulphate is : a) Blue b) Green c) Colourless d) White 2. The colour of magnesium ribbon is : a) Greyish white b) Brown c) Black d) Green 3. Magnesium oxide, when placed on moist litmus paper : a) It remains red b) It turns blue c) It becomes white d) It becomes colourless 4. The gas formed when zinc granules react with dilute sulphuric acid is : a) H2S b) H2 c) SO2 d) SO3 5. The correct observation when you mix barium chloride solution with sodium sulphate solution is that : a) A white precipitate is formed after sometime b) A yellow precipitate is formed after sometime c) A white precipitate is formed instantaneously d) A yellow precipitate is formed instantaneously 6. The colour of anhydrous copper sulphate is : a) White b) Green c) Brown d) Blue 7. BaCl2 a) b) c) d) + H2SO4 -------- BaSO4 + 2HCl is : Combination reaction Decomposition reaction Displacement reaction Double displacement reaction 8. When copper sulphate crystals are heated in a test tube, what is NOT observed during the reaction is : a) Water vapour condenses on the test tube b) A brown gas is produced c) Blue colour disappears d) A white powder is formed inside the test tube 9. We want to carry out a reaction between zinc granules with sulphuric acid. One bottle contains concentrated sulphuric acid and another bottle contains dilute sulphuric acid. The correct way of carrying out the reaction is a) Use concentrated sulphuric acid b) Add water to concentrated sulphuric acid before using it c) Use dilute sulphuric acid d) Mix concentrated and dilute sulphuric acid and add water to it 10. Identify correct statement : a) Zinc is more reactive than hydrogen b) Hydrogen is more reactive than zinc c) Zinc is more reactive than H2SO4 d) All of these Scoring Key Experiment No.2 Q.No. 1 2 3 4 5 6 7 8 9 10 Correct choice a a b b c a d b c a Explanation .Hydrated copper sulphate (CuSO4.5H2O) is blue in colour .Mg ribbon is graying white in colour Mg(OH)2 is basic in nature .Zinc displaces hydrogen from dilute H2SO4 It is an ionic reaction, therefore, precipitation occurs instantaneously. Copper sulphate lose blue colour with the loss of water of crystallisation. Barium and hydrogen have exchanged their places in the solutions Copper sulphate does not produce brown gas on heating Adding water to conc.H2SO4 is not advisable Zinc is above hydrogen in the reactivity series hence more reactive than hydrogen. SAMPLE PAPERS FOR WEEKLY TESTS WEEKLY TEST - I (2011-2012) Subject: Chemistry Grade: 9 Name: Max. Marks:13 Section: Roll No: General Instructions: This question paper consists of ?printed pages. All answers to be written in the answer sheet provided. 1. 2. 3. 4. 5. 6. State the conditions required for petroleum gas to get liquefied. Which contains more heat energy and why – 1 kg of water at 373 K or 1 kg of ice at 373 K? State your observations when : a) Ammonium chloride is heated in a hard glass test-tube. b) Carbon dioxide gas is compressed to 70 atmospheric pressure. Rohit was recording the temperature in an experiment where ice was kept for melting. He noticed that the temperature remained constant even thought a lot of ice had melted to form water. Explain why the temperature remained constant? When would Rohit observe a rise in temperature? Give reason for the following : a) Ice at 273 K is more effective in cooling than water at the same temperature. b) Liquids take the shape of the container in which they are stored. c) You feel cold after stepping out of a swimming pool . a) Convert the following temperatures : i)298 K to Celsius scale ii) – 273OC to Kelvin scale b)List the factors which affect the rate of evaporation. c)Ancient scientists believed that matter is made up of particles but they differed in one concept – some thought that the particles are continuous and some thought that they are particulate in nature. How will you prove the nature of particles in matter, with the help of an activity? Draw a neat , labeled diagram also. 1 1 1 2 1 1 1 1 1 3