Polysaccharides
advertisement

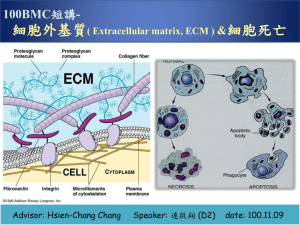
CLASS: Fundamentals- Hour 2 (11:00-12:00) DATE: 8/18/10 PROFESSOR: Pritchard POLYSACCHARIDES Scribe: FARAH BUTT Proof: CALVIN SIMS Page 1 of 5 *Note about the test: Won’t be asked to draw structures on the test. Just be familiar with how they work, and you will end up being more comfortable with them. I. II. III. IV. V. Polysaccharides [S1] a. A polysaccharide is a long chain of sugars. i. They can all be the same sugar, or they can all be different. ii. Sometimes there are units of 5 or 6 sugars repeating over and over. b. Homopolysaccharides consist of only one type of sugar. c. Heteropolysaccharides consist of more than one type of sugar. d. Glycosaminoglycans are glucose molecules that are present on all cells of our body and between cells. e. Proteoglycans are glycosaminoglycans linked to proteins. Cellulose [S2] a. Most abundant biological molecule on the planet. i. It accounts for half of the carbon in the biosphere. ii. There are a lot of trees, so there is a lot of cellulose. b. The diagram consists of a chain of glucose molecules. c. When determining if the structure is alpha or beta, look at carbons 1 and 6 (carbon 1 on the furthermost left molecule is the one that is linking the oxygen to the next molecule; carbon 6 is the one in the CH2OH group). If both of these carbon-oxygen bonds are pointing upwards (are in the same plane above the paper), then the ring is in beta configuration. If one is up and one is down, it is alpha. d. The only carbon with two oxygens attached to it is the anomeric carbon (carbon 1). e. Carbon 1 of the first molecule is connected to carbon 4 of the next molecule. f. Glucose molecule chains (and other chains that are hooked this way) form strong hydrogen bonds with other chains. i. You end up getting cellulose that is totally insoluble in water. g. Cotton is almost pure cellulose. h. Cows can digest cellulose because they have bacteria in the lumen of their stomach that make cellulases to break this down into sugar. Other animals, including humans, can’t digest cellulose. i. You can dissolve cellulose in solutions like copper. And then you can regenerate it by squirting it through a tiny circle spinneret into a solution of acid. When you regenerate cellulose, it’s called rayon. j. Diagram shows beta (1,4) over and over again of glucose. Chitin [S3] a. Second most common molecule in the biosphere. b. It is also beta (1,4), but instead of glucose, it is n-acetylglucosamine. c. Major component of crab, lobster, and shrimp shells d. There is a large amount of zooplankton in ocean, and their exoskeleton is made of chitin. e. Chitin is insoluble. f. Humans can’t digest chitin. Cows can digest it to a limited extent, but not to a very good form. g. You can make an equivalent of rayon, regenerate chitin, and make resolvable sutures out of it. This is widely used now. Dextran [S4] a. Glucose homopolymer b. The oxygen group coming off of carbon 1 is on the opposite side of the plane as the group on carbon 6. That makes it an alpha (1,6) glucan. c. The bonds are shown zig-zag, but it’s not really like that. It’s just the way it’s drawn on a flat piece of paper. d. Allene Rosalind Jeannes made a contribution to using various polysaccharides. She is famous for pioneering the use of the compound dextran as a plasma extender. e. Dextran, unlike cellulose, is extremely soluble. You can make a 60% solution out of it. It is thick as honey. f. We can’t digest dextran. g. What is a plasma extender? i. You can give people who have lost a lot of blood a transfusion of 5% dextran. A plasma extender has the effect of not only increasing the volume of blood by itself, but it tends to draw fluid out of the tissues. That effect extends the plasma volume. h. Dextran transfusions are widely used in medicine and research. Storage Polysaccharides [S5] a. Glucose homopolymer b. Amylose is soluble starch. c. Cellulose was beta (1,4) of glucose molecules, but this diagram of amylase is alpha (1,4) CLASS: Fundamentals- Hour 2 (11:00-12:00) Scribe: FARAH BUTT DATE: 8/18/10 Proof: CALVIN SIMS PROFESSOR: Pritchard POLYSACCHARIDES Page 2 of 5 d. Alpha has totally different properties than beta. e. Starch is digestible. f. People have always been able to detect starch by adding a drop of iodine to a diluted starch solution. You get a dark blue/black color in this iodine-starch reaction. VI. Amylopectin [S6] a. Long chains of alpha (1,4) linked glucose units. b. Notice that it is cross-linked. c. It is insoluble, but we can digest it because we have enzymes in our small intestine and saliva that break this down into glucose. d. There is a debranching enzyme that cleaves the branches connecting the molecules. VII. Pectin [S7] a. Don’t bother learning the structure. b. Know: Pectin is a polysaccharide in many fruits and vegetables. c. You can buy it at a grocery store. i. Used in homemade jams and jellies d. “Soluble fiber” is a horrible term for it, but it means that it is an indigestible polysaccharide. VIII. Polysaccharides Gums Commonly Added to Food [S8] a. Polysaccharides referred to as gums. b. Used in processed foods c. Average American eats about 15 grams of these gums a day. IX. Guar Gum [S9] a. Comes from beans grown in southwest Texas and New Mexico b. Common thickener c. Don’t worry about the structure of the molecule. d. Consists of a long chain of mannoses. e. Every second mannose is substituted with a galactose. f. Added to cheese, hotdogs, and salad dressings. i. To keep the liquid from running out of the cheese, companies add the gums to tie up the water. ii. We can’t digest it, but it doesn’t do us any harm. X. Xantham Gum [S10] a. Example: A bottle of Italian salad dressing has a bunch of spices floating in it. They don’t settle to the bottom because the dressing has this polysaccharide gum in it. XI. Carrageenan [S11] a. Present in dairy foods b. Just know that it is a polysaccharide gum added to foods. XII. Alginate [S12] a. Can’t digest it XIII. Carboxymethylcellulose [S13] a. You’ll find this in processed foods. b. Remember, cellulose is insoluble. If you heat that cellulose in strong alkali and treat it with monochloroacetic acid, you can put a carboxymethyl group on the C6 hydroxyl. It will have a negative charge, and the sodium is the counter-ion. Now, this molecule is soluble. c. You can make a really concentrated solution of carboxymethylcellulose. d. It is a clear, thick gooey solution. e. We can’t digest it because we don’t have an enzyme that will cut its bonds. Cellulase would cut it, but we can’t. XIV. Glycosaminoglycans [S14] a. Polysaccharides that are a major component of our cells and the matrix between all of our cells. b. There are seven glycosaminoglycans. c. Hyaluronan used to be called hyaluronic acid. Now most polysaccharides end in “an” XV. Hyaluronan [S15] a. Repeating disaccharide unit of glucuronic acid and N-acetylglucosamine b. 5 mg/mL of this would make a thick gooey solution. But, it’s actually not hard for the solution to go through a plastic syringe with a fine needle. c. Major component in aqueous humor of the eye, cartilage, and synovial fluid (in joints). d. The molecule has the property of shear dependent viscosity. i. Under high shear, the viscosity breaks down to almost zero. ii. When there is no movement, the liquid is gooey. CLASS: Fundamentals- Hour 2 (11:00-12:00) Scribe: FARAH BUTT DATE: 8/18/10 Proof: CALVIN SIMS PROFESSOR: Pritchard POLYSACCHARIDES Page 3 of 5 iii. It is an important property because the molecule takes up a lot of space and ties up water with it. It weakly immobilizes water. You don’t want a joint that is so watery. But, if you want to move your joints real fast, you want that viscosity to break down. And this is what type of property hyaluronic acid has. iv. Veterinarians inject the joints of race horses with hyalruonic acid. v. Ophthalmologists replace lost hyaluronic acid after operating on the eye by injecting it into the eyeball. XVI. Chondroitin-4-Sulfate [S16] a. Sulfate is on position 4 of N-acetyl-galactosamine (GalNac) b. People believe it helps treat arthritis, but there is no scientific proof. c. Only present in meats (especially pig’s feet), d. Maybe you don’t need it in your diet because vegetarians don’t eat meat and don’t have any more joint problems than others. XVII. Chondroitin-6-Sulfate/Dermatan Sulfate [S17] a. Difference between chondroitin sulfate and dermatan sulfate: Instead of the carboxylic acid group being above the plane of the paper (typical of a D sugar), there is an enzyme called epimerase that flips it down. Now it becomes an L sugar. It is an alpha and not a beta anymore because the bonds are on opposite sides. XVIII. Keratan Sulfate [S18] XIX. Heparin/Heparan Sulfate [S19] a. Heparin is found in certain cells of the body, as heparan sulfate is found in other places. b. They have similar structures. c. There is a sulfate group on the glucuronic acid. Chrondroitin sulfate does not have that on the glucuronic acid. d. There is a sulfate group on the amino group of the glucosamine. e. This disaccharide shows three sulfates total, but the actual amount is about 2.5 in heparin. f. Heparin is a powerful anti-coagulant. g. People can get internal bleeding from too much heparin. XX. Heparin [S20] a. Blood-clotting is a complicated process. b. Prevention of clotting: i. Protein in our blood, called fibrinogen (we have high levels of it), can be cut by an enzyme that is specific for it. ii. When cut, it forms an insoluble clear clot that is called fibrin. iii. The enzyme that cuts it is called thrombin. iv. A molecule called antithrombin III binds either weakly or not at all to thrombin. v. But when heparin binds to antithrombin III, it then binds strongly to thrombin and then inactivates thrombin so that you don’t get cleaved fibrinogen and clotting. vi. So, heparin prevents clotting by bonding to antithrombin III, which then binds more tightly to thrombin, so that you don’t get cleavage of fibrinogen. c. There is something called a surgical glue that was promoted several years ago. A surgeon would have a double syringe (two syringes together): one containing thrombin, another containing fibrinogen. The idea is to inject this into a wound, it would immediately cause the thrombin to cleave the fibrinogen, and you would get the clear natural glue of fibrin. Don’t know how well that works. XXI. Sulfated pentasaccharide sequence [S21] a. The particular arrangement of these sulfate groups is important for clotting. b. Don’t need to know the structures. Just know it’s important for clotting. XXII. Linkage of Glycosaminoglycans to Proteins [S22] a. All glycosaminoglycans, except hyaluronan, are linked through 3-sugar linkage regions to protein. b. Probably the only time you will see xylose. c. The three sugar linkage region was discovered at UAB (Gal, Gal, Xyl). d. The linkage is very alkali-labile. e. You can isolate glycosaminoglycans. You purify these sugar chains by extracting alkali. You end up breaking the bond that links it to the protein (the natural state of the molecule). Now when people look at the proteoglycans, they don’t use alkali. f. When you link a glycoaminoglycan to a protein, it’s a proteoglycan. XXIII. Keratan Sulfate Linkage in Cartilage [S23] a. Skipped XXIV. Biosynthesis of Chondroitin Sulfate [S24] a. Built up one sugar at a time. b. A protein with a serine or threonine residue is at the beginning of the reactions. CLASS: Fundamentals- Hour 2 (11:00-12:00) Scribe: FARAH BUTT DATE: 8/18/10 Proof: CALVIN SIMS PROFESSOR: Pritchard POLYSACCHARIDES Page 4 of 5 c. First you add xylose from a UDP, then galactose, then another galactose. You make the three sugar linkage region. d. Then you add glucuronic acid. e. Every so often, you add sulfate to it. XXV. Sulfation of GaINAc [S25] a. Whenever sulfate is added to a group, you’ll see that the sulfate source is always phosphoadenosine phosphosulfate. b. Sulfate and phosphate are both highly negatively charged, so they repel each other and the bond between them is easily broken. XXVI. Proteoglycans [S26] a. Can be simple (a small protein with a single chondroitin sulfate chain) or long chains (proteoglycans with hundreds of chondroitin sulfate chains). XXVII. Four Major Groups of Proteoglycans [S27] XXVIII. Hyalectans [S28] a. Given this name because they have a hyaluronic acid binding site at the N-terminus side of protein b. The blue lines are chondroitin sulfate chains. c. The lectin binding region is on the right (of the top picture). XXIX. Versican [S29] a. Read and learn it. Could ask questions from it. XXX. EM [S30] a. The size looks like 12 microns. It’s a large molecule. b. Identified: Central core that is a hyaluronic acid molecule. c. One bottle-brush is called an aggreccan. i. Each one is a separate proteoglyccan. ii. Protein chain that is bristling with chondroitin sulfate and keratin sulfate molecules. iii. Hundreds of these are linked to the long hyaluronic acid chain. d. Hyaluronic acid can have a molecular weight in millions. e. The little dots are called linked protein. i. Important for holding the aggrecan chains to the hyaluronic acid. f. Entire structure is called an aggrecan aggregate. g. Every chondroitin sulfate chain contains hundreds of sulfate groups. Sulfate groups are highly negatively charged and immobilize a lot of water. XXXI. Cartilage Proteoglycans Provide Resilience [S31] a. Its strength comes from collagen. b. Its resilience comes from the aggrecan aggregate. c. You don’t want a hard substance between your bones, so we have cartilage. d. If you don’t move your joints for a long time, they won’t get nutrients and will suffer. e. Lesson of this slide: Proteoglycans of cartilage are responsible for the resilience of cartilage. XXXII. Major Proteoglycans of Basement Membrane [S32] a. Too much info that we don’t need b. If you have two different tissues together, they are almost always separated by a basement membrane. c. The basement membrane is made up of collagen and basement membrane proteoglycans. d. A major basement membrane proteoglycan is called perlecan. e. There are certain motifs that are patterns of the amino acids in the protein part of perlecan. i. Important part of perlecan is the polysaccharide chains. XXXIII. Perlecan [S33] XXXIV. Myotendinous Junction/Perlecan at a Myotendinous Junction [S34] a. When you stain a basement membrane with perlecan, it really lights up. XXXV. Syndecans and Glypicans- Cell Surface Proteglycans [S35] a. Two major cell surface proteoglycans: syndecans and glypicans b. Glypicans are bound by GPI anchor (will find out about it later) XXXVI. Glypican/Syndecan [S36] a. Heparan sulfate chain plays important role in cell signaling and determining shape of tissues and organs. XXXVII. Syndecan [S37] a. Syndecans are embedded in the membrane and have a hydrophobic region that pulls them in. XXXVIII. Small Leucine-Rich Proteoglycans (SLRPs) [S38] a. Decorin is one of the simple SLRPs. i. It is one protein chain with a lot of leucine-rich regions (shown in green). ii. There is one single chondroitin sulfate or dermatan sulfate chain. CLASS: Fundamentals- Hour 2 (11:00-12:00) Scribe: FARAH BUTT DATE: 8/18/10 Proof: CALVIN SIMS PROFESSOR: Pritchard POLYSACCHARIDES Page 5 of 5 b. With modern genetic engineering, you can make a knockout mutant. There will be two alleles for making decorin protein, and you can knock out both alleles. In mice, they end up getting a mouse with all kinds of things wrong with it (e.g. skin easily damaged and bleeds). XXXIX. SLRP Molecules [S39] XL. Decorin [S40] XLI. Decorin Knockout mouse skin [S41] a. EM of mouse skin b. The collagen fiber cross-sections are the circles. c. In normal mouse skin, all of the collagen fibers are about the same size. d. In decorin knockout mice, you get big collagen fibers. e. Decorin plays an important role in the assembly of collagen fibrils. XLII. Model of Human Decorin [S42] XLIII. Mucopolysaccharidoses [S43] a. You have to not only make proteoglycans, but you also break them down. Our body is constantly making and breaking things. b. Breakdown occurs in lysosome. It is loaded with proteolytic enzymes that break down protein, lipids, or glycosaminoglycans. c. If you lack one of these enzymes to break it down, you will have mucopolysaccharidoses. It is not that uncommon (1 in 30,000). i. Most often leads to mental retardation. XLIV. Diseases [S44] a. Don’t memorize this. Just see that there are diseases that can occur when you lack certain enzymes needed for the breakdown of glycosaminoglycans. [End 45:01 mins]