Hexagonal HoMnO3 oxide HoMnO3 becomes magnetically ordered
advertisement

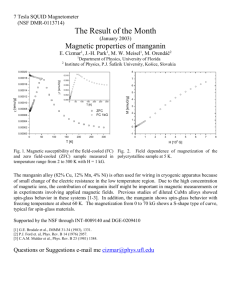
STRUCTURAL AND MAGNETIC PROPERTIES OF MECHANICALLY ACTIVATED OXIDE HoMnO3 V. Mitrofanov, G. Kozhina, O. Fedorova Institute of Metallurgy of UB RAS, 101, Amundsen st., Ekaterinburg, Russia vyam@mail.ru Introduction Manganese oxides of the general formula RMnO3 (R = rare-earth element) have been intensely studied in recent years owing to the exciting properties as the colossal magnetoresistance (CMR) and multiferroic effects [1]. Pure polycrystalline hexagonal multiferroic HoMnO3 (hHoMnO3) can be synthesized by the conventional solid-state reaction method. However, h-HoMnO3 can be converted to the corresponding orthorhombic perovskite phase (o-HoMnO3) through a high pressure treatment. Hexagonal HoMnO3 oxide becomes magnetically ordered at TN ≈ 72 K, and another two magnetic transitions take place at lower temperatures [1-8]. Neutron powder diffraction measurements demonstrate that, below the ordering temperature TN, the moments of the Mn3+ cations adopt a triangular spin arrangement, the magnetic moments lying in the basal plane and parallel to the [100] axis [2]. At TSR = (32-45) K [1-8], the moments reorientate within the basal plane and become aligned perpendicularly to the initial direction [2]. Mn3+ spin rotation is accompanied by a partial ordering of the Ho3+ spins [2, 9, 10]. The magnetic moment of the Ho3+ ion is completely ordered below T(Ho3+) 5 K combined with another rotation of Mn spins in the basal plane [10]. Considerable variation in the TSR values is associated with the choice of initial reagents, synthesis conditions of single crystals and ceramic materials [1-8]. The magnetic properties and the low-temperature magnetic structures of the orthorhombic perovskite o-HoMnO3 have been studied on polycrystalline samples by A. Muñoz et al [11]. It was shown that oHoMnO3 exhibits three singularities at TN =41 K, T1 26 K, and T2 6.5 K. Below TN = 41 K, the Mn3+ magnetic moments become ordered in an antiferromagnetic arrangement, adopting a modulated sinusoidal magnetic structure. At T1 26 K a transition to a commensurate 117 magnetic structure takes place. Ordered Ho3+ magnetic moments appears below 9 K. Size effect on magnetic properties of hexagonal HoMnO3 nanoparticles was systematically studied in [8]. The magnetic data indicates that with increasing the particle size, the antiferromagnetic (AFM) transition temperature TN increases from 50 to 70 K, whereas with a decrease in particle size, the Mn-spin reorientation temperature TSR is enhanced from 39 to 43 K. It is well known that mechanoactivation of inorganic solids is a useful tool for the preparation of metastable crystalline and amorphous phases, and also for nanostructured materials not-obtainable through conventional methods [12-15]. It may be noted some of the advantages of this method such as shortening of reaction times, reduction of the high temperatures commonly required for solid-state reactions and the possibility of producing materials with special properties [14]. The study of various properties of mechanically activated (MA) oxides allows us to determine the influence of the activation process on the structure, defects, distribution of cations over nonequivalent sublattices, the charge and the orbital states of the ions, the magnetic properties of 3d-oxides. One of the results of intensive mechanical activation is the appearance of nanosized states. It is well known that when the size of the material is reduced to the nanometer scale the magnetic properties can vary significantly. Reducing the size of the crystallites can lead to a decrease of temperatures of magnetic phase transitions, the saturation magnetization, remanent magnetization, coercivity. Currently there is no information in the literature on the effect of mechanical activation on the magnetic properties of HoMnO3. Investigation of the properties of these MA oxides can significantly complement the relevant data on the magnetic characteristics of nanostructured oxide systems obtained by different methods. Experimental procedure Polycrystalline samples of HoMnO3 were synthesized by the conventional solid-state reaction method, using Ho2O3 and Mn2O3 (company Sigma-Aldrich) with purity of 99.9 % as starting materials. Stoichiometric mixtures of these compounds were annealed in air at 1400 ◦C for 96 h in a muffle furnace Nabertherm NT 04/16. Mechanical treatment of single-phase samples HoMnO3 was carried out in planar type stainless118 steel mill AGO-2 for 60, 90, 120 and 300 seconds. The X-ray diffraction (XRD) patterns of all samples were obtained by a Shimadzu XRD-7000 diffractometer using Cu K radiation in the 2 range of 20-70 deg with exposition at each point for 2 seconds. The magnetic properties of HoMnO3 compounds were examined on vibrating sample magnetometer Cryogenic VSM CFS-9T-CVTI. Magnetisation data were collected between 5 and 300 K at a magnetic fields of 0.01 and 0.015 T under both zero-field-cooled (ZFC) and field-cooled (FC) modes. The field dependencies of magnetization were obtained at the constant temperatures of 5 K, 10 K, 20 K, 30 K, 50 K and 80 K in the magnetic fields up to 2 T. Measurements of the magnetic properties were carried out on the following samples: the initial polycrystalline sample HoMnO3, MA samples with grinding times 60 and 300 seconds. Experimental results and discussion Fig. 1a shows the powder XRD pattern of the initial HoMnO3 samples. Based on the standard reference, all the observed peaks can be indexed on the basis of a hexagonal unit cell of space group P63cm, suggesting that the sample is pure phases without any impurity. Crystal lattice parameters: a = 6,138 (3) Å, c = 11,412 (7) Å, V = 372,3 Å3. All MA HoMnO3 samples contain two phases. Along with the main phase having a hexagonal structure, in the diffraction patterns were also present reflexes of HoMnO3 phase with orthorhombic structure (space group Pbnm). Content of orthorhombic phase increases with increasing duration of mechanical activation. As a result, the lattice parameters of the hexagonal phase are changed (Table 1). In the study of the influence of mechanical activation in highenergy ball mill AGO-2 on the structural characteristics of HoMnO3 sample it was found that a sharp decrease in the average size of coherent scattering regions (CSR) up to 30 times occurs in the first 60 seconds of grinding. The microstrain value practically does not change with increasing time of mechanical treatment, reaching values of 0.50 % for the duration of mechanical activation from 90 s to 300 s (see Table 1 and Fig. 2). 119 Fig. 1. The x-ray diffraction patterns of the HoMnO3 samples: а – initial; b – mechanoactivated in the mill AGO-2 for 300 s (arrows indicate the reflections of the orthorhombic phase). Table 1. Structural characteristics of MA oxides HoMnO3 Time of mechanical activation, s 0 The lattice parameters с, Å The unit cell volume V, Å3 The CSR mean size D, nm 6,138(3) 11,413(7) 0,3723 622 The mean value of microstrain ε, % 0 а, Å 60 6,142(3) 11,419(8) 0,3731 20 0,6 90 6,137(3) 11,411(4) 0,3722 24 0,5 120 6,137(6) 11,416(7) 0,3724 34 0,48 300 6,130(6) 11,415(8) 0,3715 20 0,5 120 Fig.2. Dependence of the mean size of coherent scattering region and microstrains on the time of grinding for HoMnO3 samples. Fig. 3 shows the ZFC and FC curves of the initial HoMnO3 crystal measured at a magnetic field H = 0.01 T. In the upper inset, we have shown FC curve in the low-temperature region bellow 5.5 K. Fig. 3. ZFC and FC curves for the initial HoMnO3 sample under a magnetic field of 0.01 T. Inset: FC magnetization in the low-temperature region. 121 Near about 5 K the FC curve shows an anomaly with a sudden increase in magnetization with decreasing T. This abrupt change corresponds to the antiferromagnetic (AFM) ordering of Ho3+ moments at TN(Ho3+)5 K. A second anomaly is observed at TSR 45 K below which the FC and ZFC curves strongly bifurcate. The change of the magnetization at T = TSR is an indication that at least some of the spins of Ho3+ ions are AFM ordered, and this ordering is linked with the transition of re-orientation of the spins of Mn3+ ions. At a temperatures TN 76-89 K, the ordering of the moments of Mn3+ ions takes place. For a better illustration of the magnetization peculiarities, the results for the initial HoMnO3 sample are presented in Fig. 4. The sharpness of the two magnetic transitions is obvious from the two narrow peaks that appear in the derivative, d(H/M)/dT, at TSR =45 K and TN =76 K for the FC mode, TSR =45 K and TN =89 K for the ZFC mode. Fig. 4. Temperature dependences of the magnetization derivative d(H/M)dT for the initial HoMnO3 sample. The inverse magnetic susceptibility data exhibit linear behavior above 100 K. The Curie-Weiss temperature (θcw) and effective magnetic moment (ef ) were calculated based on linear parts according to the Curie-Weiss fitting. The respective θcw value is −17 K, implying the existence of antiferromagnetic ordering below the TN . The experimental ef122 fective magnetic moment ef=10.5 B, which is slightly lower of the theoretically expected value of 11.7 B. The values obtained for the temperature TN ,TSR, TN(Ho3+)and θcw confirms the results by other authors [1-8]. In Fig. 5 and Fig. 6 temperature variations of FC and ZFC magnetizations under a magnetic field of 0.015 T and 0.01 T, respectively, for the sample obtained by the mechanical grinding are shown. The divergence between the FC and ZFC curves may be explained in terms of the superparamagnetism or suggesting that the as-milled samples contain impurities with a ferromagnetic component having the Curie temperature higher than the room temperature. Fig. 5. ZFC and FC curves for the MA HoMnO3 sample in the mill AGO-2 for 60 s (S1) under a magnetic field of 0.015 T. We must refer to contamination which may bring about the ferromagnetic behavior observed, because the ball milling process causes the contamination by some impurities from the balls and the container (steel) used for milling. Absence of no clear magnetic transition peaks at T<100 K in the M versus T curves can be explained as the suppression due to the large paramagnetic susceptibility of the Ho3+ ions [2] and ferromagnetic impurities from the balls and the container of the mill AGO2. With decreasing temperature, a rapid increase of magnetization gives transition points in magnetic characteristics of samples. To determine 123 these points more accurately, the derivative of magnetizations with respect to temperature are given in Fig. 7 and Fig. 8. Fig. 6. ZFC and FC curves for the MA HoMnO3 sample in the mill AGO-2 for 300 s (S2) under a magnetic field of 0.010 T. Fig. 7. The temperature derivative of magnetization vs. temperature for S1 sample. 124 Fig. 8. The temperature derivative of magnetization vs. temperature for S2 sample. The observed anomalies in the temperature dependences dM(T)/dT of the MA samples S1 and S2 at T 7, 9, 12, 15 K can be related to the ordering of the holmium ions, spin-reorientation transitions in Mn sublattice in h-HoMnO3 and-HoMnO3 phases. The isothermal magnetization (M-H) curves of the initial and MA samples HoMnO3 measured at 5, 10, 20, 30, 50 and 80 K 10 K are shown in Fig. 9-11. Fig.9. Representative M-H isotherms of the initial HoMnO3 sample measured at different temperatures. 125 Fig.10. Representative M-H isotherms of MA S1 sample measured at different temperatures. Fig.11. Representative M-H isotherms of MA S2 sample measured at different temperatures The isotherms vary almost linearly with increasing magnetic field with the field in the low-field region. The value of magnetization of MA 126 samples at H =2T and T = 5 K reduces significantly with a decrease of size, which might be attributed to the increasing of surface to volume (S/V) ratio, since the surface region leads to a decrease in the effective magnetic moment. Temperature dependence of coercive force (HC) for HoMnO3 compounds are shown in Fig. 12. Fig.12. Temperature dependence of coercive force (HC) for HoMnO3 compounds Nonmonotonic dependence of HC for the initial sample is associated with antiferromagnetic ordering of holmium at T 5 K and spinreorientation transition at TSR 45 K in the manganese sublattice. The presence of ferromagnetic impurities with ordering temperature T> 300 K in the MA samples HoMnO3 is confirmed by increase in Hc with increasing of grinding time and the weak dependence of Hc vs. T in the temperature range 30-80 K. Conclusion 1. The data on the magnetic ordering temperatures in the initial HoMnO3 are in good agreement with literature data. Established essential irreversibility of ZFC and FC curves below the temperature of spinreorientation transitions in Mn sublattice of h-HoMnO3 (45 K), a nonmonotonic temperature dependence of coercivity Hc. 127 2. Irreversibility (bifurcation) of ZFC and FC curves of MA samples above TN(Mn) is associated with the superparamagnetic nanoparticles HoMnO3 and the presence of ferromagnetic impurities material of balls and glass mill (iron or iron oxides). 3. Found the reduction of TN (Mn) of MA sample (60 s) and the magnetization M at H = 2 T and T = 5 K of MA samples is due to the growing influence of the surface of the nanoparticles. 4. The observed anomalies in the temperature dependences dM(T)/dT of samples MA at T 7, 9, 12, 15 K likely to be associated with the ordering of the holmium ions, spin-reorientation transitions in Mn sublattice in h-HoMnO3 and-HoMnO3 phases. 5. The presence of ferromagnetic impurities with ordering temperatures > 300 K in the MA samples HoMnO3 is confirmed by increase in Hc with increasing of grinding time and the weak dependence of Hc vs. T in the temperature range 30-80 K Acknowledgements The work was supported by the Program of the Presidium of Russian Academy of Sciences, project number 12-P-3-1021. Experimental results were obtained using the instruments of the Collective Equipment Center “Ural-M”. 1. 2. 3. 4. References W.Prellier, M. P. Singh and P Murugavel. The single-phase multiferroic oxides: from bulk to thin film// J. Phys.: Condens. Matter. 2005. V. 17. P. R803–R832. A. Muñoz, J. A. Alonso, M. J. Martínez-Lope, M. T. Casáis, J. L. Martínez, and M. T. Fernández-Díaz. Evolution of the Magnetic Structure of Hexagonal HoMnO3 from Neutron Powder Diffraction Data// Chem. Mater. 2001. V. 13 (5). P. 1497–1505. E. Galstyan, B. Lorenz, K. S. Martirosyan, F. Yen, Y. Y. Sun, M. M. Gospodinov and C.W. Chu. Magnetic hysteretic phenomena in multiferroic HoMnO3 single crystals and polycrystals with nanoand micrometer particle size// J. Phys.: Condens. Matter. 2008. V. 20. P. 325241 (7pp). A. Midya, S. N. Das, P. Mandal, S. Pandya and V. Ganesan. Anisotropic magnetic properties and giant magnetocaloric effect in 128 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. antiferromagnetic RMnO3 crystals (R=Dy, Tb, Ho and Yb). arXiv:1111.6739v1 [cond-mat.str-el] 29 Nov 2011. K. Uusi-Esko, J. Malm, N. Imamura, H. Yamauchi, M. Karppinen. Characterization of RMnO3 (R = Sc, Y, Dy-Lu): High-pressure synthesized metastable perovskites and their hexagonal precursor phases// Materials Chemistry and Physics. 2008. V.112. P. 1029– 1034. I. Radulov, V. Lovchinov, D. Dimitrov, P. Simeonova, Ph. Vanderbemden. Magnetic and transport properties of HoMnO3 monocrystals// Journal of optoelectronics and advanced materials. 2009, V. 11 (7), P. 1553 – 1556. A. Midya, P. Mandal, S. Das, S. Banerjee, L. S. Sharath Chandra, V. Ganesan, and S. Roy Barman. Magnetocaloric effect in HoMnO3 crystal// Appl. Phys. Lett. 2010. V. 96. P. 142514 (3 pp). T. C. Han, M. R. Tsai, and C. Y. Wei. Size effect on magnetic properties of hexagonal HoMnO3 nanoparticles// J. Appl. Phys. 2011. V.109. P. 07B517 (3 pp). T. Lottermoser, T. Lonkai, U. Amann, D. Hohlwein, J. Ihringer and M. Fiebig. Magnetic phase control by an electric field// Nature. 2004. V. 430. P. 541-544. F. Yen, C. dela Cruz, B. Lorenz, E. Galstyan, Y. Y. Sun, M. Gospodinov, and C.W.Chu. Magnetic Phase Diagrams of Multiferroic Hexagonal RMnO3 (R=Er, Yb, Tm, and Ho)//J. Mater. Research. 2007. V. 22. P. 2163-2173. A. Muñoz, M. T. Casáis, J. A. Alonso, M. J. Martínez-Lope, J. L. Martínez, and M. T. Fernández-Díaz. Complex Magnetism and Magnetic Structures of the Metastable HoMnO3 Perovskite//Inorg. Chem. 2001. V. 40. P. 1020-1028. V. V. Boldyrev, K. Tkacova. Mechanochemistry of solids: Past, Present and Prospect.// Journal of Materials Synthesis and Processing. 2000.V.8. P. 121-132. C. Suryanarayana. Mechanical alloying and milling.// Prog. Mater. Sci. 2001. V. 46. P. 1-184. D. L. Zhang, Processing of Advanced Materials Using Highenergy Milling// Prog. Mater. Scie. 2004. V 49. P. 537-560. C. Suryanarayana. Recent Developments in mechanical alloying. Rev. Adv. Mat. Sci. 2008. V. 18. P. 203-211. 129