IHC Considerations:
advertisement

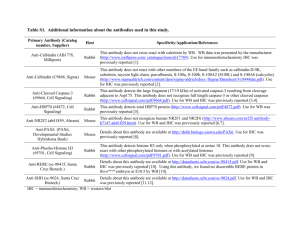
IHC Core Guidelines and Instructions Page 1 of 4 IHC Considerations: An antibody that works for ELISA, Western or Flow Cytometry may not work for IHC. If the antibody has not been tested with IHC before, it will take quite a bit of effort to determine if the antibody can be used for IHC---optimizing will be necessary for good results. There should be a plan: Do you have tissue as negative and positive controls? What will be the range of antibody dilutions and incubation times? What will be the right blocking reagents? What will be the secondary reagents? Which antigen retrieval method? Heat induced antigen retrieval with different pH or enzyme digestion? Will a fluorescence OR enzyme detection system be used? Will catalyzed signal amplification be needed? Other Questions: 1. Are you going to immunostain frozen sections or paraffin sections? 2. What type of antibody is your primary antibody? A polyclonal rabbit? Polyclonal goat? Mouse monoclonal or rat monoclonal? 3. What is the tissue you are testing: human or mouse tissue? From what organ? 4. Is the antibody commercially available and tested and shown to work well in immunohistochemistry assays? 5. What is the expected Positive control tissue for the antibody? 6. What is the expected Negative control tissue for the antibody? 7. Do you have cells in tissue culture that you know are positive or negative for the antibody that can be used as controls in the assay? 8. If the antibody has never been tested in IHC, what is the concentration of antibody that you used on Westerns or ELISA to obtain the positive staining? IHC Core Guidelines and Instructions Page 2 of 4 IHC Core Guidelines SLIDE POSITIONING: Each slide contains 3 staining zones. The following total volumes of reagent are recommended: 3 zones – 250 µL 2 zones – 200 µL 1 zone – 150 µL 1 2 3 Case # Protocol Zone #: Note: The volumes may need to be increased for large human tissues. For optimal results, the sections should be centered within each zone. To save slides and reagents, we recommend placing experimental and control tissues on the same slide for the same antibody. (The autostainer cannot stain different antibodies on the same slide.) See examples of tissue arrangement on the slides below: Case # Protocol Block ID Case # Protocol Block ID KO#1 KO#2 WT#1 WT#2 positive ctrl negative ctrl experimental PARAFFIN SECTIONS: 4-5 microns Deparaffinization: Slides should be dried overnight 37°C post-sectioning and prior to deparaffinization, heated in oven at 60°C for 30-45 min in a horizontal position. Submerge slide in Slide Brite® (preferred safe non-hazardous method): 5min/change x 3 changes OR Submerge slide in Xylene: 10 min/change x 2 changes IHC Core Guidelines and Instructions Page 3 of 4 Continue as below: Rehydrate: 1. 100% ethanol 1 min 2. 95% ethanol 1 min – 2x 3. 70% ethanol 1 min 4. Leave in DI water until staining Keep the slides in the DI water until ready to perform antigen retrieval. At no time from this point onward should the slides be allowed to dry. Drying out will cause non-specific antibody binding and therefore high background staining. Deparaffinization should be done the night before or in the morning before you drop off your slides. FROZEN SECTIONS: 5 microns Slides should be left to air dry overnight at room temperature immediately after sectioning. If slides are not used the next day, wrap in foil and store in -20°C freezer until use. Fixation: If slides are stored in the freezer, let the slides come to room temperature before removing the foil. 1. Dip slides in Acetone: absolute alcohol (3:1) for 10-15 min at room temperature 2. Immediately dip slides in TBS buffer 3. Keep slides in buffer until ready to stain Slides from this point should not be allowed to dry. This step should be done immediately before you send the slides to the core lab. The following steps will be done in the IHC core lab: A. Antigen Retrieval – Decloaking chamber (for paraffin sections) Rodent decloaker – heat retrieval solution for rodent tissues ~ 95°C for 30-60 min ~ 120-125°C for 30 sec/5 min Diva decloaker - heat retrieval solution for human tissues 95-99°C for 30-40 min Antigen retrieval for delicate tissues can use a lower temperature setting for a longer period. IHC Core Guidelines and Instructions Page 4 of 4 B. Block endogenous peroxidase Peroxidazed 1 C. Protein block Human - Background Sniper Mouse – Rodent Block M D. Immunostaining Choosing the right reagent: Mouse-on-mouse HRP polymer kit Rat-on-mouse HRP polymer kit Rabbit-on-rodent HRP polymer kit MicromerTM Universal HRP Detection kit (for mouse anti-human or rabbit anti-human antibodies) UltraVision LP Detection System – HRP polymer & DAB Plus Chromogen The following chromogen kits are available: DAB (including if desired DAB Sparkle – DAB post-enhancing solution) Romulin AEC Fast Red E. Counterstaining Hematoxylin (Blue, nuclei) We will leave the slides in DI water for you to pick up. You can mount your slides as follows: G. Dehydration/Mounting 1. 70% ethanol 1 min. 2. 95% ethanol 1 min – 2x 3. 100% ethanol 1 min. 4. Xylene 10 min – 2x OR Slide Brite 2 min – 3x 5. Air dry 6. Coverslip