protocol, a FORM - Dalhousie University
advertisement

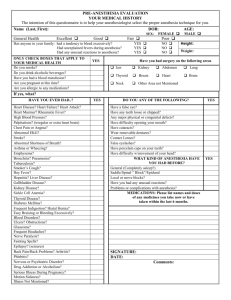
Jan 2013 FORM A UNIVERSITY COMMITTEE ON LABORATORY ANIMALS APPLICATION TO USE ANIMALS FOR RESEARCH or TEACHING - ETHICS REVIEW Principal Investigator (must have Faculty appointment): Faculty Position: Building: Department: Phone: E-Mail: Project Title: Renewal of Previous Protocol? Yes: Is this protocol for: Research: No: Teaching: Is the proposed work currently funded? Yes: Previous Protocol Number: Testing: No: Pending: Name of funding source(s): Have ALL the concepts for the experiments proposed in this protocol be peer reviewed for scientific merit? Yes: No: Pending: Who provided the peer review? All protocols must be reviewed for scientific merit. If this project has not been reviewed, or was reviewed but not funded, please see APPENDIX 2. Is this protocol a contract or part of a contract? Proposed Start Date: Yes: No: Proposed End Date: EMERGENCY CONTACTS: MUST include phone numbers for PRINCIPAL INVESTIGATOR and ONE OTHER PERSON designated to handle animal emergencies or media inquiries. Must be available for evenings and weekends. Name Certificate # Department Position After hours phone 1. 2. DECLARATION: I certify that all animals used in this research project will be cared for in accordance with the recommendations of the Canadian Council on Animal Care and the policies of Dalhousie University. I assume responsibility for providing each member of the laboratory who will perform procedures under this protocol with a copy of the final version of the approved protocol, and require that they follow the procedures described. I will notify the UCLA of any changes to the proposed project before proceeding with any animal experimentation. SIGNATURE: DATE: Principal investigator or course director Page 1 of 9 Jan 2013 SECTION 2 RESEARCH STAFF: Principal Investigator MUST have a current certification number. Add additional personnel on a separate sheet. Name Certificate # Department Position Phone 1. 2. 3. 4. 5. RESEARCH PERSONNEL TRAINING: Everyone handling laboratory animals is required to be listed as research staff and have a certification number in addition to practical training for the species they are using (see Certification/Training in information section, or contact the University Veterinarian’s office for more information). Add additional personnel on a separate sheet. For research staff required to handle live animals, check off which workshops/training have been attended. Acronym description of practical workshops currently offered is provided below: NAME RH MH ANES AS FH Custom Session Describe below * Other Describe below * 1. 2. 3. 4. 5. Describe CUSTOM SESSION with Lab Animal Training Coordinator or OTHER Training here: RH = Rat Handling : Recommended Tech Procedures Familiarization with handing, restraint, injection and blood collection procedures in the in the rat. MH = Mouse Handlng : Recommended Tech Procedures Familiarization with handling, restraint, injection and blood collection procedures in the in the mouse ANES = Intro to Anesthesia Principles of Anesthesia AS = Intro to Aseptic Surgical Technique Aseptic surgical tech, post-op care, analgesia, scrub, gown, glove tech, suture FH = Fish Handling and Water Quality Evaluation of water quality parameters. Anesthesia, tagging, blood collection & surgical techniques Custom Session = Individualized sessions with Dalhousie Lab Animal Training Coordinator Other = eg. formal training from other institutions, or training from other qualified personnel Will anyone listed on the protocol require new/extra training for some procedures? If “yes”, please describe what procedures: Yes No Page 2 of 9 Jan 2013 SECTION 3: Project Objectives: Briefly describe the objectives of the proposed work using animals. SECTION 4: What is the potential benefit that might result from your research? If applicable, please indicate the disease process that this work will investigate. SECTION 5 (PART 1) CHECKLIST: This YES / NO checklist will provide assistance in determining which Attachments will be required. Check YES or NO for each item below, and complete the required attachment for each YES. 5a. Does this project involve surgery and/or administration of anesthetics? YES NO If yes , complete and include ATTACHMENT A It is NOT necessary to complete Attachment A if an anesthetic overdose is being administered solely as a method of euthanasia, without any surgical manipulations, recordings, etc. performed on anesthetized animals. 5b. Is this protocol a renewal of a previously approved protocol? YES NO If yes, complete and include ATTACHMENT B (NOTE: Attachment B is different from Form B) 5c. Will these animals be used for teaching? If yes, complete and include ATTACHMENT C YES NO 5d. Is this a field study? If yes, complete and include ATTACHMENT D YES NO 5e. Are agents hazardous to humans or animals, such as radioisotopes, biohazardous materials, chemicals or radiation administered to animals? YES If yes, complete and include ATTACHMENT E NO 5f. Is breeding required as part of this protocol? YES NO If yes, complete and include ATTACHMENT F All breeding colonies must be covered under an approved protocol. Breeding animals can be included as part of this research protocol by completing Attachment F. Alternatively, if many breeding colonies are maintained, or if a breeding colony supports many different experimental protocols, a separate breeding protocol can be submitted. 5g. Are animals being immunized in this project? If yes, complete and include ATTACHMENT G 5h. Does this project involve the injection or implantation of tumours, stem cells or other biological materials (other than tissue transplants) into rodents? If yes, complete and include ATTACHMENT H YES NO YES NO 5i. Does this project involve safety/efficacy/toxicity testing for regulatory purposes? If yes, complete and include ATTACHMENT I YES NO 5j. Does this project involve removing live animals from the animal facility and YES taking them to another location? (For example, taking them to your laboratory or to and imaging suite) If yes, complete and include ATTACHMENT J. NO Page 3 of 9 Jan 2013 SECTION 5 (PART 2): PROJECT DESCRIPTION Use lay language Do not excerpt pages from a grant application Provide a detailed description of all procedures to be performed in animals (note that details of surgeries should be described in attachment A) Include information on anticipated effects of these procedures on the animals Use a Flow Chart(s) to indicate what happens to animals at different time points Include group sizes in the flow chart (s) If applicable, include your own SOPs or SOPs from our website at http://animalethics.dal.ca. Contact Leslie at 494-1270 for login information. “ (this answer section will expand) SECTION 6: The Three R’s The three Rs (replacement, reduction and refinement) are the cornerstone of ethical animal research, and the UCLA requires investigators to implement the 3Rs when they are preparing to use animals for scientific purposes. Please show how you have considered the 3Rs in your project design and in your answers to the following questions: 6a. Justify the number of animals based on the experimental design of this project. Indicate how the number of animals was determined by breaking their use down into experimental groups. Include a statistical justification if possible, or a yield of tissue needed per animal. (this answer section will expand) 6b. Is this the minimum number of animals that can be used to obtain valid results? 6c. This protocol is valid for a maximum of TWO years. Indicate the number of animals you will use EACH year. If additional animals are required before the expiry date of this protocol, a FORM B may be submitted requesting additional animals. SPECIES STRAIN WEIGHT or AGE SEX # Required in Year 1 # Required in Year 2 Total Animals Required SUPPLIER Page 4 of 9 Jan 2013 6d. 6e. If animals for this study are produced by in house breeding, you must include ATTACHMENT F or provide the number of your currently approved breeding protocol. PROTOCOL # ANIMAL HOLDING FACILITY: BUILDING: ROOM: EXPERIMENTAL STUDIES: ROOM: BUILDING: 6f. Why must animals be used in this study? If alternatives to animals are available, indicate why they cannot be used in this study. 6h. Explain choice of animal model or animals species. 6i. If you are using more than one rodent strain, why? Please explain why each strain was chosen, and how the use of the strains relates to your objective. 6j. Can normal environmental enrichment devices be provided to captive animals on this study? YES NO If NO, please justify withholding these items. Page 5 of 9 Jan 2013 SECTION 7 : HUMANE ENDPOINTS Some experimental manipulations or phenotype abnormalities can be expected to produce a degree of unavoidable pain, distress or illness in experimental animals. CCAC guidelines require that adverse effects Abe minimized or alleviated by choosing the earliest endpoint consistent with the scientific objectives of the research. 7a. What is the expected time course of the study? (ie., how long are animals maintained from the first experimental manipulation until the end of the experiment or planned euthanasia?) For field studies what is the period of observation? 7b. Do you expect this study to cause any pain, distress or illness? YES NO If yes, please describe, including time course. (this answer section will expand) 7c. What clinical signs / observations may indicate that animals used in this project are experiencing pain, distress or illness? (e.g. behavioural changes such as decreased grooming, vocalization or postural changes, or physical abnormalities such as anorexia, dehydration, diarrhea, etc.) (this answer section will expand) When are these signs expected to occur and when would they be most severe? (this answer section will expand) 7d. Animals must be monitored by research staff throughout the course of the experiment. Monitoring records must be kept. Any unexpected morbidity or mortality must be reported to the veterinary staff. How frequently will animals be monitored during the course of the study as well as during the critical times when signs are most severe? (this answer section will expand) Who will monitor the animals? (this answer section will expand) Page 6 of 9 Jan 2013 7e. What criteria, appropriate to the species, will trigger the decision to end the study, stop the procedure, or humanely euthanize an animal before the experimental objective is achieved? (examples could include the following: a weight loss limit as a percentage of body weight, allowable durations of anorexia, ulcerative skin lesions). (this answer section will expand) 7f. The veterinarian has the authority to euthanize animals in distress; however, a reasonable attempt will be made to communicate with the research group before taking action. Who in the research group can authorize the euthanasia of animals? SECTION 8 - CCAC CATEGORIES OF INVASIVENESS Using APPENDIX 1 (For wildlife use APPENDIX 1B) indicate the Level of Invasiveness that best identifies this protocol. B C D E For LEVEL A experiments - the use of invertebrates (excluding higher invertebrates eg. all cephalopods), eggs, protozoa or other single-celled organisms, tissue obtained from sources such as a slaughterhouse or from another source, please use a Form C. PURPOSE OF ANIMAL USE: 0. 1. 2. 3. 4. 5. This is a CCAC requirement. Please indicate which single statement BEST describes the purpose of animal use in this protocol. Breeding ONLY Studies of a fundamental nature in sciences relating to essential structure or function(e.g. biology, psychology, biochemistry, pharmacology, physiology). Studies for medical purposes, including veterinary medicine, that relate to human or animal diseases or disorders Studies for regulatory testing of products, for the protection of humans, animals, or the environment. Studies for the development of products or appliances for human or veterinary medicine. Education and training of individuals in post-secondary institutions or facilities CCAC Animal Care Classification Category: An acute study is one in which animals are euthanized without an extended holding period or prior invasive manipulations, unless these are performed under anesthesia without recovery. This would include animals euthanized for tissue collection, or anesthetized for a surgical procedure and euthanized before waking up. A chronic study involves experimental manipulations on conscious animals, or recovery from anesthesia. Is this study ACUTE or CHRONIC ? Page 7 of 9 Jan 2013 SECTION 9 - EUTHANASIA 9a. METHODS OF EUTHANASIA SPECIES ACCEPTABLE METHODS DRUG ROUTE DOSE Anesthesia overdose Anesthesia with subsequent CO2 Anesthesia with subsequent exsanguination Anesthesia with subsequent decapitation Anesthesia with subsequent cervical dislocation SPECIES CONDITIONALLY ACCEPTABLE METHODS DRUG ROUTE DOSE N/A N/A N/A N/A N/A N/A N/A N/A N/A decapitation WITHOUT sedation or anesthesia (see 9b) cervical dislocation WITHOUT sedation or anesthesia (see 9b) CO2 Chamber WITHOUT sedation or anesthesia (see 9b) Other method (please specify and also see 9b) 9b. IMPORTANT: If a conditionally acceptable method of euthanasia was indicated above, provide scientific justification (with references where available) for why sedation or anesthesia cannot be used: (this answer section will expand) Describe the training or experience of personnel performing the conditionally acceptable method of euthanasia: (this answer section will expand) 9c. If animals are not to be euthanized at the completion of the study, please indicate what will happen to them: Page 8 of 9 Jan 2013 10. YES The use of controlled drugs (such as ketamine, buprenorphine and sodium pentobarbital) requires an exemption from Health Canada. Have you applied for/or received an exemption? NO NOT APPLICABLE Page 9 of 9