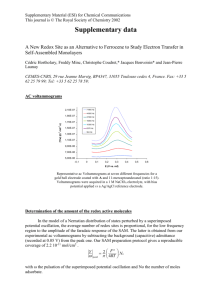
TEM images and cyclic voltammograms
advertisement

# Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2003 Supplementary Information: S-1 shows the TEM image of the MSA capped Au nanoparticles. The synthesis of the MSAGNP followed the method described by S. Chen and K. Kimura (S. Chen and K. Kimura, Langmuir, 1999, 15, 1075), which was based on the reduction of hydrogen tetrachloroaurate(Ш) by sodium borohydride in methanol using MSA as the stabilizing thiol ligand. The particle size can be easily controlled by the initial molar ratio of MSA/HAuCl4. In our case, the molar ratio is 1:1. TEM image shows that the MSAGNP are well-seperated, and the average particle size is about 2 0.5 nm. S-2 shows the cyclic voltammograms of PANI/MSAGNP measured in buffers with different pH values: for pH 1, HCl was used; for pH 3~8, 0.1M citrate phosphate buffer was used. Scan rate was 20mV/s. In low pH solution, two separate redox peaks appear, as the pH increases, the second redox peak moves quickly toward low potentials with a rate of about –100mV/pH and finally merges with the first one at pH > 5 to show only one redox peak. Similar phenomena were also observed for PANI doped by other methods( see references 7,9,11). S-3 shows the cyclic voltammograms of different bilayers of PANI/MSAGNP measured in 0.1M PBS buffer, pH 7.1 with and without 5mM NADH. The inset shows that the electrocatalytic efficiency of the film increases with the increase of the number of bilayers up to at least 12 bilayers. # Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2003 S-1 TEM image of the MSA capped Au nanoparticles. 4 I / µA 0 -4 pH=3 pH=1 pH=5 -8 pH=7 -12 pH=8 -0,2 0,0 0,2 0,4 E/V 0,6 0,8 S-2 cyclic voltammograms of PANI/MSAGNP measured in buffers with different pH values: for pH 1, HCl was used; for pH 3~8, 0.1M citrate phosphate buffer was used. Scan rate 20mV/s. 12 8 anodic catalytic current / µA # Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2003 7 with 5mM NADH 6 5 4 2 2 4 I / µA 12 10 8 6 4 2 3 4 6 8 10 number of bilayers 12 0 2 4 6 8 10 12 -4 -8 -0,2 -0,1 0,0 0,1 0,2 0,3 E/V S-3 Cyclic voltammograms of different bilayers of PANI/MSAGNP measured in 0.1M PBS buffer, pH 7.1 with and without 5mM NADH. The inset shows the relationship between the electrocatalytic ability and the number of bilayers.