NSAID Prescribing Guidance July 13 v2
advertisement

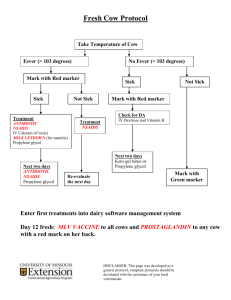
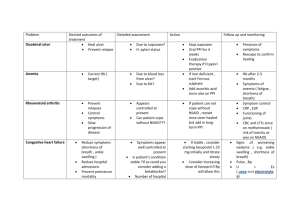
`ADVICE TO PRESCRIBERS NON STEROIDAL ANTI INFLAMMATORY DRUGS What are the risks of non steroidal anti inflammatory drugs (NSAIDs)? All NSAIDs are associated with gastrointestinal (GI), cardiovascular (CV) and renal side effects. Diclofenac is contra-indicated in all patients with ischaemic heart disease; peripheral arterial disease; cerebrovascular disease; or established congestive heart failure (New York Heart Association [NYHA] classification II–IV) Paracetamol (and/or topical NSAIDs) is recommended ahead of oral NSAIDs in the NICE osteoarthritis guideline1. If NSAIDs are required, they should be prescribed at the lowest effective dose for the shortest period of time necessary to control symptoms2. Following a European medicines Agency review of etoricoxib® the MHRA recommends3 that: o Patients whose blood pressure is persistently above 140/90 mmHg and inadequately controlled must not receive etoricoxib o High blood pressure should be controlled before starting treatment with etoricoxib and should be monitored for two weeks after the start of treatment and regularly thereafter. How can the GI risks of NSAIDs be reduced? NICE guidance states that a proton pump inhibitor (PPI) should routinely be co-prescribed with an NSAID (including coxibs) for anyone with osteoarthritis1 or rheumatoid arthritis4 and anyone 45 years of age and older with chronic low back pain5, choosing the PPI with the lowest acquisition cost. A hospital based case control study showed a marked increase in risk of upper GI ulcer bleeding with NSAIDs overall, but use of a PPI attenuated the risk. There was no increased GI risk of NSAIDs plus a PPI over non-use of an NSAID6. There is no good evidence to suggest that when NSAIDs are given with a PPI there are any differences between NSAIDs in terms of GI risk. How can the CV risks of NSAIDs be reduced? If an NSAID is required, CV risk can be reduced by using low dose ibuprofen (1200 mg/day or less) or naproxen (1000 mg/day) rather than other NSAIDs for patients in whom CV risk is a significant consideration in decision making.2 Coxibs (celecoxib and etoricoxib) are associated with an increased thrombotic risk7 Diclofenac (150 mg/day) has a similar excess thrombotic risk to etoricoxib and possibly other coxibs The European Medicines Agency has recently started a new review of the CV risks of NSAIDs8 How can the renal risks of NSAIDs be reduced? Patients at risk of renal impairment or renal failure (particularly the elderly) should avoid NSAIDs if possible9. If NSAID treatment is absolutely necessary, the lowest effective dose for the shortest possible duration should be used and renal function should be carefully monitored10 What about the link between PPI use and C Diff Infections? PPI use has been linked to an increased risk of C. Diff infection11. The value of using a coprescribed PPI to reduce gastric insult needs to be balanced against the increased risk of D:\106740046.doc C. Diff infection observed with PPI use. Twice daily doses of PPIs should be avoided as the risk is significantly greater than once daily dosing. Local Advice The appropriateness of NSAID prescribing should be reviewed widely and on a routine basis. This is especially the case for people at high risk of both GI and CV morbidity and mortality (e.g. older patients). If NSAIDs are required, steps should be taken as outlined above to reduce their GI, CV and renal risks. As a result, most prescribing of NSAIDs should be for low dose ibuprofen and naproxen. If initiating an NSAID is essential use ibuprofen (1200 mg /day or less) or naproxen (1000 mg/day or less). Review patients currently prescribed NSAIDs according to the local Stockport procedure. If continued use is necessary, consider changing to ibuprofen (1200mg/day or less) or naproxen (1000mg/day or less). Review and, where appropriate, revise prescribing of etoricoxib to ensure it is in line with MHRA advice and the NICE clinical guideline on osteoarthritis. Co-prescribe a proton pump inhibitor (PPI) with NSAIDs for people with osteoarthritis, rheumatoid arthritis or low back pain (for people over 45 years) where regular use will be needed for longer than 6 months. For short term use < 1 month a PPI is not required unless there is a significant other clinical risk of gastric upset. Take account of drug interactions when co-prescribing NSAIDs with other medicines (see Summaries of Product Characteristics). For example, co-prescribing NSAIDs with ACE inhibitors or angiotensin receptor blockers (ARBs) may pose particular risks to renal function; this combination should especially be carefully considered and regularly monitored if continued. Use of SSRIs with NSAID will increase the risk of bleeding. For moderate duration (i.e. 1-6 months) the prescribing clinician needs to make a risk assessment in relation to risk of GI bleed including concomitant use of other GI irritant medication such as SSRI antidepressants. This would indicate an increased need for PPI cover however previous C diff infection would make the use of a PPI less desirable due to an increased risk of re-infection. Therefore a case by case assessment by a clinician is needed. Adapted from MeReC bulletin Volume 22 number 3 Jan 2012: http://www.npc.nhs.uk/merec/therap/other/merec_bulletin_vol22_no3.php 1 NICE. Osteoarthritis. The care and management of osteoarthritis in adults. Clinical guideline 59. February 2008 NPC. Cardiovascular and gastrointestinal safety of NSAIDs. MeReC Extra 30. November 2007 3 MHRA. Etoricoxib: new prescribing guidelines. Drug Safety Update. July 2008 4 NICE. Rheumatoid arthritis. The management of rheumatoid arthritis in adults. Clinical guideline 79. February 2009 5 NICE. Low back pain. Early management of persistent non-specific low back pain. Clinical guideline 88. May 2009. 6 Lanas A, García-Rodríguez LA, Arroyoet MT, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclooxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut 2006;55:1731– 38 7 MHRA. Safety of selective and non-selective NSAIDs. October 2006 8 European Medicines Agency starts new review of cardiovascular risks of non-selective NSAIDs. EMA press release 21/10/11 9 European Medicines Agency starts new review of cardiovascular risks of non-selective NSAIDs. EMA press release 21/10/11 10 MHRA. Non-steroidal anti-inflammatory drugs: reminder on renal failure and impairment. Drug Safety Update. May 2009 11 NPC MeRec rapid review Increased risk of C difficile infections and of fractures: two more good reasons to review PPI prescribing http://www.npc.nhs.uk/rapidreview/?p=1494 2 Last reviewed July 13 Next Planed review July 15 D:\106740046.doc