**NOTICE: In order for this course to be considered a laboratory
advertisement

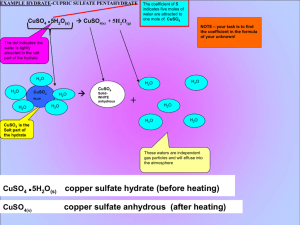
**NOTICE: In order for this course to be considered a laboratory science course (collegeprep), you must complete a minimum of 2/3 of the projects. Any incomplete projects will receive a grade of 0%, which will significantly lower your overall course grade.” Lab 8: Empirical Formula of a Hydrate Student Name: Date Experiment done: Date Report Completed: Name of Grader: Hypothesis: A prediction of what you think the results of the project will be. Write your hypothesis before you begin the experiment and, if the experiment has more than one part, you will need a hypothesis for each section. Remember to create an ‘if-then’ statement. (Example: If students get adequate rest, then grades will improve). Refer to the first Project of the semester or to the Student References Section if you need additional information (1-2 sentences). Procedure: In 1st person past tense, write a summary of what you actually did in performing the experiment. Be sure to include any modifications. You should include enough detail so that someone could reproduce the experiment based on what you have written. Data: Describe all observations or changes. List all measurements and include proper labels. Mass of empty crucible: Mass of crucible and CuSO4 xH2O (hydrate): Mass of crucible and CuSO4 (pure salt): Mass o f crucible: Mass of crucible and MgCl2 x H2O (hydrate): Mass of crucible and MgCl2 (pure salt): Calculations/Interpretations: Show all math performed (label the calculation or give the formula, show your setup, and give the result), include proper labels, and/or answer any questions listed below. Mass of CuSO4 xH2O: Mass of CuSO4: Mass of H2O: Moles of CuSO4: Moles of H2O: Moles of H2O for every mole of CuSO4: Chemical formula of CuSO4 XH2O (Hint: replace the X with the number of moles of water for every mole of CuSO4): Mass of MgCl2 xH2O: Mass of MgCl2: Mass of H2O: Moles of MgCl2: Moles of H2O: Moles of H2O for every mole of MgCl2: Chemical formula of MgCl2 XH2O (Hint: replace the X with the number of moles of water for every mole of MgCl2): Conclusion: Begin by stating whether the hypothesis was true or false. Use data and calculations to support your answer. Consider the following questions as you write your conclusion: Why or what happened to result in the outcome you observed? Did you learn anything new? If not, what previously concepts did this lab reinforce? Is there anything you would or could do differently that would improve the experiment? Do you have any other comments/observations you would like to share about this lab? (*Note: Not all questions pertain to each lab.) Your conclusion should be approximately one paragraph in length. Trait Rate Grade Grammar / Spelling /5 / 10 Hypothesis /5 / 10 Procedures /5 / 10 Data / Observations /5 / 20 Calculations / Interpretation /5 / 20 Conclusion / Basic Understanding /5 / 30 Total A